Background and Objectives: Serratus fascial plane block can reduce pain following breast surgery, but the question of whether to inject the local anesthetic superficial or deep to the serratus muscle has not been answered. This cohort study compares the analgesic benefits of superficial versus deep serratus plane blocks in ambulatory breast cancer surgery patients at Women’s College Hospital between February 2014 and December 2016. Wetested the joint hypothesis that deep serratus block is noninferior to su
perficial serratus block for postoperative in-hospital (pre-discharge) opioid consumption and pain severity.
Methods: One hundred sixty-six patients were propensity matched among 2 groups (83/group): superficial and deep serratus blocks. The co hort was used to evaluate the effect of blocks on postoperative oral morphine equivalent consumption and area under the curve for rest pain scores. We considered deep serratus block to be non inferior to superficial serratus block if it were non inferior for both outcomes, within 15 mg morphine and 4 cm·h units margins. Other outcomes included intra- operative fentanyl requirements, time to first analgesic request, recovery room stay, and incidence of postoperative nausea and vomiting.
Results: Deep serratus block was associated with postoperative morphine consumption and pain scores area under the curve that were noninferior to those of the superficial serratus block. Intraoperative fentanyl require ments, time to first analgesic request, recovery room stay, and postoperative nausea and vomiting were not different between blocks.
Conclusions: The postoperative in-hospital analgesia associated with deep serratus block is as effective (within an acceptable margin) as superficial serratus block following ambulatory breast cancer surgery. These new findings are important to inform both current clinical practices and future prospective studies.
(Reg Anesth Pain Med 2018;43: 480–487)
The single-injection serratus fascial plane block has recently gained popularity because of its analgesic efficacy for breast cancer surgery,1–6 relative safety, and the ease with which it is learned, taught, and performed.7 Described by Blanco and colleagues,8 the block targets the lateral cutaneous branches of the intercostal nerves as they traverse either of 2 fascial planes: superficial to the serratus muscle or deep to the serratus muscles. Although both the superficial and deep approaches produced sensory blockade, Blanco et al8 concluded that the superficial plane is more effective based on radiographic and sensory observations in 4 volunteers; however, these observations may not accurately reflect the true analgesic effects in surgical patients.9 Indeed, effective analgesia, along with theoretical technical advantages, has been reported with each of these 2 block techniques for a variety of chest wall and thoracic surgeries.1–5,8,10–15
Our use of the superficial serratus plane block has posed several practical concerns to the breast oncologic surgeons, including (i) spread into the axilla, thus disrupting the surgical tissue planes; (ii) block of the long thoracic and thoracodorsal nerves,8 thus interfering with surgeons’ effort to identify these nerves (tweaking by forceps)16 to preserve them; and (iii) needling through potentially metastatic lymph nodes, theoretically increasing the risk of tumorseeding.17–19 By contrast, the deep serratus plane block performed more posteriorly, along the posterior axillary line, seems to mitigate these concerns.
Our anecdotal experience 6 suggests that the 2 block techniques are similarly effective in providing postoperative analgesia following breast cancer surgery. However, whether the deep serratus block is indeed as good as the original superficial serratus block remainsun known, as the 2 techniques haven ever been compared. This propensity-matched cohort study aims to demonstrate that the deep serratus technique is as effective as the superficial serratus technique in providing postoperative analgesia following ambulatory breast cancer surgery. We tested the joint hypothesis that the deep serratus block is noninferior (with an acceptable margin) to the superficial serratus block for (i) postoperative in hospital (predischarge) opioid consumption and (ii) area under the curve (AUC) for in-hospital rest pain scores.
The authors adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines 2 in preparing this article.
This propensity-matched retrospective cohort study was approved by the Research Ethics Board at Women’s College Hospital (WCH), a University of Toronto–affiliated ambulatory hospital. The primary sources of data were the medical records of women who underwent ambulatory breast cancer surgery with general anesthesia (GA) between February 2014 and December 2016 at WCH. The requirement for written informed consent was waived as this study was limited to preexisting data that had been collected as part of the care standard. The primary data sources were individual patient hospital records and the block room logbook that captured the details of all nerve blocks administered. The data sets for women undergoing all types of breast cancer surgery at WCH during the time period of interest were retrieved, and the relevant data were extracted.
Cohort History
Our experience with the serratus block dates back to February 2014, when we introduced the superficial serratus technique described by Blanco et al8 as an analgesic adjunct for breast cancer surgery at WCH. However, the block was discontinued in October 2014, following breast oncologic surgeons’ complaints that the local anesthetic injectate often disrupts the anatomical planes in the axilla, interferes with axillary surgical interventions, and impedes their effort to preserve the long thoracic and
thoracodorsal nerves. These were coupled with a concern that the superficial serratus block involves needling through potentially metastatic lymph nodes. The superficial serratus technique was replaced by the deep serratus technique. Since then, the superficial serratus block is no longer in use at our institution.
Eligibility Criteria
All females who had breast tumor resection under GA with the addition of the superficial or deep serratus blocks performed preoperatively between February 2014 and December 2016 were considered for this study. Patients 18 to 75 years of age with a body mass index (BMI) of less than 40 kg/m2 and an American Society of Anesthesiologists (ASA) classification I to II having unilateral or bilateral breast tumor resection and receiving multi modal analgesia were considered eligible. Those with significant psychiatric history (eg, generalized anxiety disorder or major de pression), with preexisting chronic pain (lasting >3 months),21 who were opioid dependent (mean daily use of 30 mg oxycodone or equivalent per day), or with preplanned overnight hospital stay were excluded. Surgical procedures considered eligible were mastectomy alone or with sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND), and partial mastectomy with SLNB or ALND, performed by any 1 of 4 breast on cologic surgeons. For patients who underwent multiple surgeries during our study period, only the first eligible procedure was in cluded. All breast reconstruction procedures, alone or with con current tumor resection, were excluded. We also excluded all patients who did not receive the standardized preoperative oral analgesics regimen (see Care Standard) and those who received intraoperative local anesthetic infiltration by the surgeons.
Care Standard
The in-hospital analgesic management for breast cancer surgery at WCH is outlined by a clinical pathway that includes oral acetaminophen 1 g and celecoxib 400 mg administered prior to surgery and a preprinted medication order sheet for postoperative
analgesia. While variations in intraoperative management exist among anesthesiologists in the same center, all patients having breast tumor resection at WCH routinely receive GA using inhalational gas (desflurane or sevoflurane in a 50:50 air-oxygen mixture) with either a laryngeal mask airway or an endotrachealtube. Intravenous (IV) fentanyl and morphine or hydromorphone are administered for analgesia intraoperatively based on hemodynamic responses to surgical stimuli. Intravenous ondansetron 4 mgand dexamethasone 8 mg are administered intraoperatively to all patients for postoperative nausea and vomiting (PONV) pro phylaxis. Recovery room nursesassess postoperative pain severity and administer postoperative analgesics according to our institutional care standard, whereby patients requesting analgesia or reporting a visual analogscale (VAS) score(0= nopain,10=worst
pain imaginable) of 4 or greater postoperatively receive IV fentanyl (25–50 μg every 5 minutes, as needed, up to 100 μg), followed by IV hydro morphone (0.2–0.4 mg every 5 min, as needed, up to 2mg),orI V morphine (2–4mgevery5minutes,as needed,up to 10 mg), and/or oral oxycodone (5–10 mg every 2 hours, as needed). The opioid-based analgesic regimen is routinely combined with either a superficial or deep serratus block.
After having met once institutional discharge criteria, based on the modified Aldrete score,22 all women having breast cancer surgery are discharged home on the same day from phase II recovery.
Block Techniques
Both superficial and deep serratus blocks were performed preoperatively by staff anesthesiologists or supervised regional anesthesia fellows. Patients were placed in the lateral position and received midazolam 1 to3 mg IVfor anxiolysis prior to block administration. The local anaesthetic solution injected varied be tween 20 and 25 mL of ropivacaine 0.33% to 0.5%;the choice of local anesthetic volume and concentration was left to the discretion of the individual anesthesiologists. The ultrasound-guided superficial serratus block was performed in the midaxillary line, at the T4 level in the fascial plane between the latissimus dorsi muscle and serratus muscle, as previously described by Blanco et al 8 (Fig. 1). The deep serratus block was performed in a more posterior location, along the posterior axillary line, at the T4 level, in the fascial plane between the serratus anterior muscle and external intercostal muscle. With support from our oncologic surgeons, the deep serratus technique was introduced because it does not result in local anesthetic spread into the surgical planes and also avoids needling through potentially metastatic lymph nodes.
Study Design
Patients who received the deep serratus block were matched to those who received a superficial serratus block on a 1:1 ratio using propensity score matching.23 This matching was used toobtain groups of patients corresponding to the 2 analgesic modalities that are balanced with regard to potential preidentified confound
ing variables. We used a nonparsimonious multivariable logistic regression model to calculate propensity scores, in which we se lected the mode of analgesia as the dependent variable. The inde pendent variables included 8 confounders and/or predictors for acute postoperative pain following breast tumor resection, as iden
tified in the literature24,25 or perceived by the authors, including (i) age, (ii) BMI, (iii) type of surgical procedure, (iv) identity of the surgeon, (v) duration of surgical procedure, (vi) preoperative opioid consumption, (vii) local anesthetic dose, and (viii) total du ration of postanesthesia care unit (PACU) stay. Continuous con founders (age, BMI, duration of procedure, preoperative opioid consumption, local anesthetic dose, duration of PACU stay) were modeled using restricted cubic splines with 5 knots (at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles), whereas exact matching was used for categorical confounders (type of surgical procedure and identity of the surgeon). Propensity matching was performed using a 1:1 nearest-neighbor algorithm. We set the boundaries for pairs matching to a caliper size of 0.2 SDs of the logit of the propensity score, as suggested by Austin.26 We excluded patients in whom primary outcomes data were missing, or if the prespecified predictors of acute pain data were lacking.
We evaluated the balance of the matched cohort on the 8 preidentified
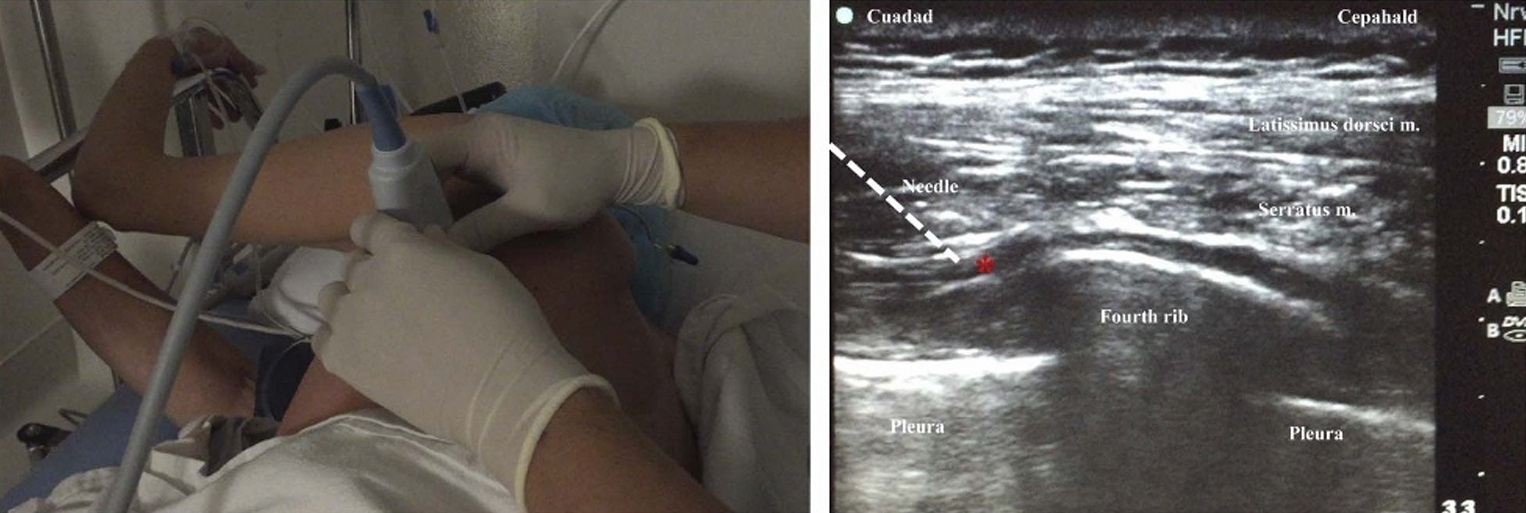
FIGURE 1. Parasagittal ultrasound-guided in-plane deep serratus block technique along the posterior axillary line. Red star designates the targeted fascial plane. White line represents the needle trajectory.
confounders after inverse weighting of these variables by the respective propensity scores; standardized differences in the matched sample that exceeded 0.1 were considered indicative of residual imbalance. All subsequent analyses were restricted to the propensity-matched patients.
Outcomes
The set of matched patients was compared for 2 primaryout comes: (i) cumulative postoperative in-hospital (predischarge) opioid consumption, as measured by oral morphine equivalents (in milligrams),27 and (ii) the AUC for postoperative rest pain severity VAS scores during their in-hospital stay. Eight pain scores corresponding to PACU phase I admission and discharge, PACU phase II admission and discharge, and best and worst pain scores documented during the stay in each of PACU I and II were considered representative of the efficacy of the patient’s pain control during their in-hospital recovery course. Both analgesic out comes were considered as mutually confirmatory evidence of the analgesic efficacy of the fascial blocks and were thus examined in conjunction. We therefore sequentially tested the primary joint hypothesis that the deep serratus block is noninferior to the superficial serratus block for the reduction in the postoperative oral morphine equivalent consumption and subsequently for the AUC of postoperative rest pain severity scores. Secondary outcomes included intraoperative opioid require ments(converted to micrograms of IV fentanyl),27 time to first analgesic request (in minutes), rate of PONV (definedaspatient self report and/or the administration of a rescue antiemetic in PACU), and duration of stay in phases I and II recovery (in minutes). In addition, we sought complications that were block related, such as local anesthetic systemic toxicity, pneumothorax, bleeding, hematoma formation, and paresthesia. Demographic data (age, ASA classification, weight, height); preoperative medications; comorbidities; history of chronic opioid use; type, duration, and laterality of breast surgery; the identity of the surgeon; and the local anesthetic dose used for the block were also collected.
Statistical Analysis
The 2 primary outcomes, postoperative in-hospital opioid consumption and AUC of rest pain scores, were modeled using multivariable regression within the propensity-matched cohort. We examined the 2 primary outcomes in a sequential manner,28 using a serial gate-keeping approach,29 beginning with opioid consumption. Specifically, if opioid consumption was first proven to be noninferior, then AUCs for rest pain scores were subsequently compared. Such serial testing is clinically justified as reduced rest pain corroborates effective analgesia only if opioid consumption is also reduced. This approach allowed the threshold of statistical significance for the noninferiority testing
to be maintained at 0.025 for the 1-tailed comparison over each primary outcome.
We used multivariable regression to model opioid consumption within the propensity-matched cohort, using a log transformation to model opioid consumption. The exponentiated difference in means for this outcome on the log scale (ie, ratio of geometric means) was subsequently used to compare the confidence inter
vals (CIs) for testing noninferiority.
We considered the deep serratus block to be noninferior to superficial serratus block if it were noninferior for both outcomes, beginning with opioid consumption, and subsequently the AUC for pain scores, with noninferiority margins (Δ) of 15 mg in oral morphine equivalent consumption and 4.0 cm·h absolute difference in the AUC. One-sided noninferiority testing for the primary out comes was performed by comparing the 95% CI of the difference between groups for the opioid consumption and the rest pain
AUCoutcomes,respectively, tothe predetermined Δ for the seout comes. Subsequently, and after demonstrating noninferiority for an outcome, we moved on to test for superiority for this outcome as well, as part of the secondary hypothesis testing. Because it is possible to claim superiority if demonstrated for either of the 2 primary outcomes, we set the threshold of statistical significance for superiority testing at 0.025. We used the χ 2, Fisher exact, Student t, or Mann-Whitney Utest, as appropriate, for comparing secondary outcomes. All time to-event outcomes were compared using the Kaplan-Meier analysis and the log-rank test. The R statistical software version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria) and SAS statistical soft ware version 9.3 (SAS Institute, Cary, North Carolina) were used in for statistical analyses. The SAS g-match macro was used for the propensity score matching.
Sample Size Calculation
We based our power analysis for this retrospective cohort on the noninferiority comparison of the cumulative opioid con sumption and ensured adequate power for examining the AUC of rest pain scores, the second primary outcome. Based on a
sample of 20 consecutive patients who received a superficial serratus block in 2014 for their breast cancer surgery at WCH, we observed a cumulative postoperative oral morphine equivalent consumption of 40 ± 22 mg. A 30-mg difference in oral morphine consumption (or 10 mg IV morphine) is generally considered as the minimum that is clinically meaningful.6 Therefore, for a 1-sided noninferiority test that designates a 15-mg difference in oral morphine consumption (or 5 mg IV morphine) as a nonin feriority margin, a type I error estimate of 0.025, and 90% power, we estimated that at least 46 patients per group were required. Our preliminary 20-patient sample who received the superficial serratus block had an AUC of rest pain severity scores of 9.2 ± 5.8 cm·h for the 8 measurements of interest. The minimum clinically important difference in pain scores is estimated to be 1.0 to 1.3 cm on a VAS scale for a single measurement.30,31 We considered a difference of 0.5 cm to be indicative of noninferiority; this is equivalent to a noninferiority margin of 4.0 cm·h for an AUC for the 8 measurements. The aforementioned sample size (46 patients per group) estimate provides 90% power to perform a 1-sided noninferiority test of the second primary outcome, AU Cofthein-hospital rest pain severity scores, considering an allowable difference of 0 mg and a type I error estimate of 0.025.
All charts of women who had breast cancer surgery during the time period of interest were screened. The review identified 493 potentially relevant records of patients who had their breast surgeries performed between February 2014 and December 2016. Of these, 97 records did not meet the inclusion criteria (Fig. 2), and another 39 records were excluded because of missing data, preplanned hospital admission, secondsame-sitesurgery(re excision or reconstruction), or surgery without inhalational GA.
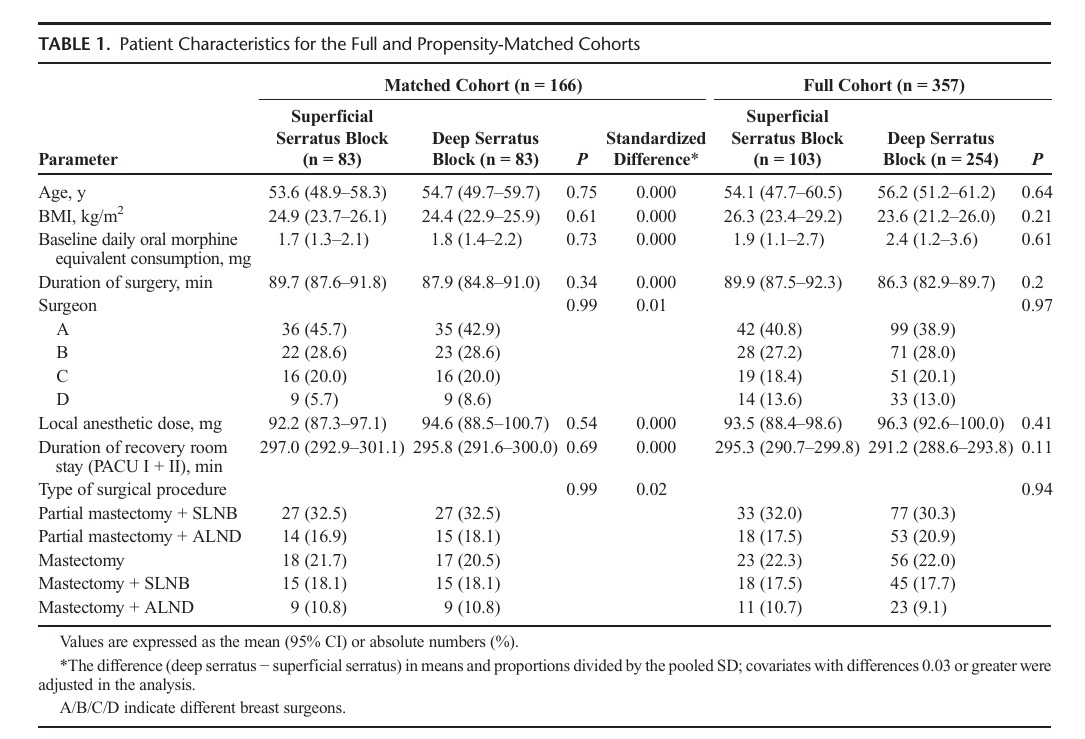
Complete data were available for the 357 records that remained (superficial serratus = 103, deep serratus = 254), constituting the full cohort. Of these, 166 were successfully matched on a 1:1 basis based on predetermined confounders and baseline characteristics, including 83 patients for each of the deep and superficial serratus blocks. The propensity-matched cohort included 80.6% and 32.7% of eligible superficial and deep serratus block patients, respectively. We considered the matched cohort to be sufficient as it pro vided 99% power for the intended tests of noninferiority. Table 1 summarizes the baseline and demographic characteristics for the full and propensity-matched cohorts. The patients in the matched
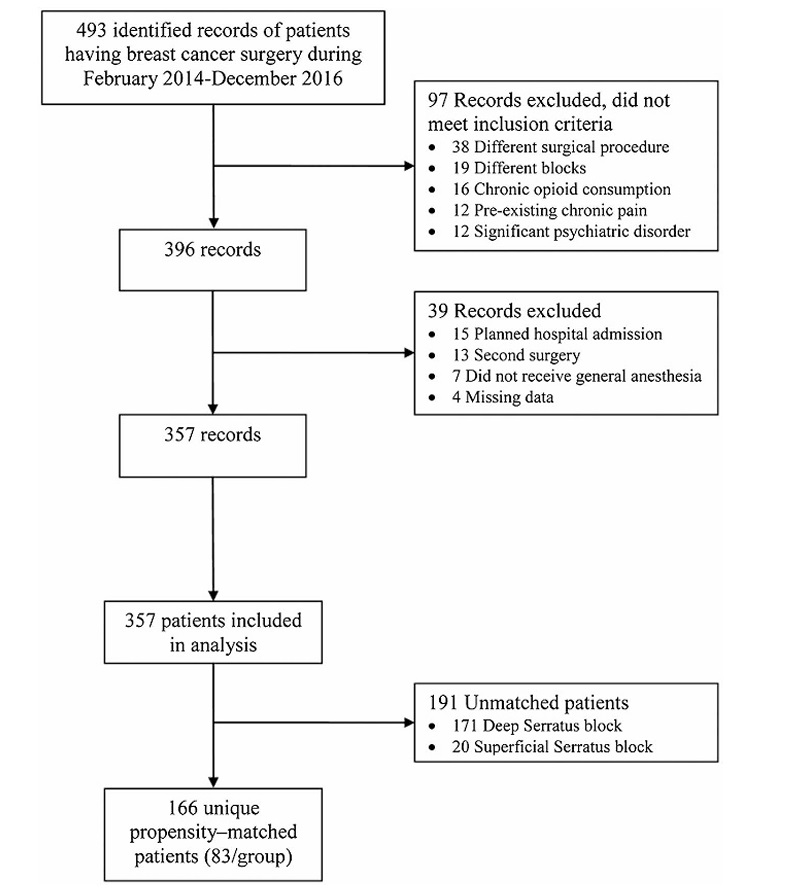
FIGURE 2. Flow diagram of case selection.
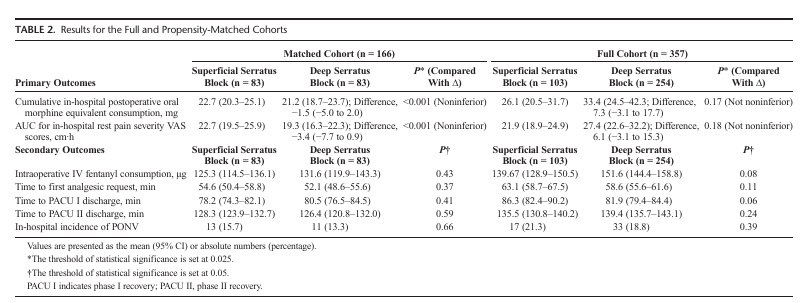
cohort had similar baseline and demographic characteristics;but the differences between the 2 groups were clinically important, albeit not statistically significant,in the full cohort,thus supporting our decision to perform propensity matching. Expressed as a me an (95%CI),the postoperative in-hospital cumulative oral morphine equivalent consumption associated with the superficial and deep serratus blocks for the matched cohortwas22.7mg(20.3–25.1mg)and21.2mg(18.7 23.7mg), respectively. The difference between the 2 blocks (deep serratus−su perficialserratus)was−1.5mg(−5.0to2.0mg),with the upper CI being significantly smaller (P<0.001) than the predesignated noninferiority margin (Δ=15mg).Our matched cohort findings suggested that the deep serratus block was noninferior but not superior to the superficial serratus block inreducing in-hospital cumulative oral morphine consumption following breast cancer surgery (Table2,Fig.3). Incontrast, the difference i npostoperative in-hospital cumulative oral morphine equivalent consumption associated with the superficial and deep serratus blocks for the full cohort was7.3mg(−3.1to17.7mg), with the upper CI crossing the predesignated noninferiority margin.The full cohort findings suggested that the deep serratus was not noninferior to the superficial serratus block for in-hospital cumulative oral morphine consumption following breast cancer surgery (Table2).
The AUC of the postoperative in-hospital rest pain VAS scores associated with the superficial and deep serratus blocks for the matched cohort was 22.7cm·h (19.5–25.9cm·h) and 19.3cm·h(16.3–22.3cm·h),respectively(Fig.4).The difference bet ween the 2 blocks (deep serratus−superficial serratus) was −3.4cm·h(−7.7to0.9cm·h), with the upper CI being significantly smaller (P<0.001) than the predesignated noninferiority
margin (Δ=4cm·h).Our matched cohor tfindings suggested that the deep serratus block was noninferi or but not superior to the superficial serratus block inproviding in-hospital restpainre lief following breast cancer surgery (Table2,Fig.3).
In contrast, the differencein the AUC ofthe in-hospital rest pain VAS scores associated with the superficial and deep serratus blocks for the full cohort was 6.1cm·h (−3.1to15.3cm·h),with the upper CI crossing the predesignated noninferiority margin. The full cohort findings suggested that the deep serratus was not noninferior to the superficial serratus block for the AUC of the postoperative in-hospitalrestpa in VAS scores(Table2).
Both the full and matched cohorts suggested that the superficial and deep serratus blocks were not different in terms of the intraoperative fentanyl consumption, time to first postoperative analgesic request, time to discharge from phase I and phase II PACU, and the incidence of in-hospital PONV (Table2).None of the patients included in this cohort experienced any block-related complications;and none of the patients needed unplanned extended hospital stay or admission.
Our results suggest that the superficial and deep serratus techniques are similarly effective in reducing postoperative in hospital opioid consumption and controlling in-hospital postoperative pain following ambulatory breast cancer surgery. Notably, the other analgesic outcomes associated with these 2 techniques, such as intraoperative fentanyl consumption,time to first analgesic request, duration of stay in phases I and II recovery,and incidence of PONV,were not different bet ween the superficial and deep serratus blocks.
Despite the inherent shortcomings of retrospective data, there are several reasons why the present results are novel, important, and informative. First, no trials have previously compared these 2 block techniques.While proponents of the“original”superficial serratus block have described successful analgesia with out the potentially hazardous need for advancing the needle deeper toward the pleura,3,5,8,10–13 thoughtful consideration of
anatomy arguably favors the deep serratus block as injection in the fascial plane below the serratus muscle theoretically facilitates blockade of higher-order lateral cutaneous intercostal nerves,which are known for their extensive ramification and collateral branching. 32
Our current findings encourage the transition to the deep serratus block incenters seeking alternatives for the superficial serratus block. Breast oncologic surgeons performing axillary interventions (SLN Bor ALND ) on patients who received superficial serratus block have observed local anesthetic fluid infiltration and disruption of the axillary tissue planes, particularly around these level I lymph nodes (or lateral axillary lymph node group),which are the primarysurgical target for patients under going axillary biopsy or dissection. 33,34 The senodes may be locate don the anteromedial surfaces of the latissimus dorsi or subscapularis muscle or the lateral surface of the serratus
muscle 35 and may belocated a long the needle trajectory when superficial serratus block is administered.The local anesthetic spread following the superficial serratus block also blocked the long thoracic and thoracodorsal nerves,interfering with the onco- logic surgeons’ intraoperative stimulation of these nerves proximally and theef fort toidentifyandpreservethem.16 In contrast, the surgeons have motivated the transition to the deep serratus block, as this technique seems toad dress the aforementioned
surgical concerns.
The propensity-matching technique we used to account for biases yielded differences in the primary outcomes between the matched and full cohorts; such differences have 2 plausible explanations. First, matching causes some sample attrition because of the inherent trade-off between the quality of matching and the resultant number of matched patients. 36,37 Indeed, 191 patients in the full cohort were not matched and were excluded from analysis, which may have contributed to the observed differences. Second, the 8 covariates on which the sample was matched are established risk factors for acute postoperative pain 24,25; thus, the observed differences in the matched cohort are reflective of real differences after ajusting for biases.
There are several limitations to this cohort study.The observational design permits drawing conclusions regarding potential associations,and not causal relationships. However,a retrospective study that examines the impact of practice change is the only
feasible design, as the superficial serratus block has already been discontinued at our institution.The latter notwithstanding, the 2 interventions examined herein were used in tandem (deep serratus block replaced the superficial serratus block ) rather than simulta
neously , and were cognize that the transition between the 2 techniques during ourstudy period may have been accompanied by other subtle changes in clinical practice that we did not detect. For example,we can not control for the possibility that some anes the siologists and nurses had more prolonged exposure to the deep serratus technique, which may have influenced their opioid administering practices. Moreover, although propensity score matching may have reduced the risk of bias and improved the validity of our results, we were able to account for confounders that we could identify only if the relevant data were obtainable.For example, the effect of certain acute pain predictors, such as anxiety, catastrophization, ethnicity, genetics, and socioeconomic factors,
would have been interesting to examine, but data relating to these
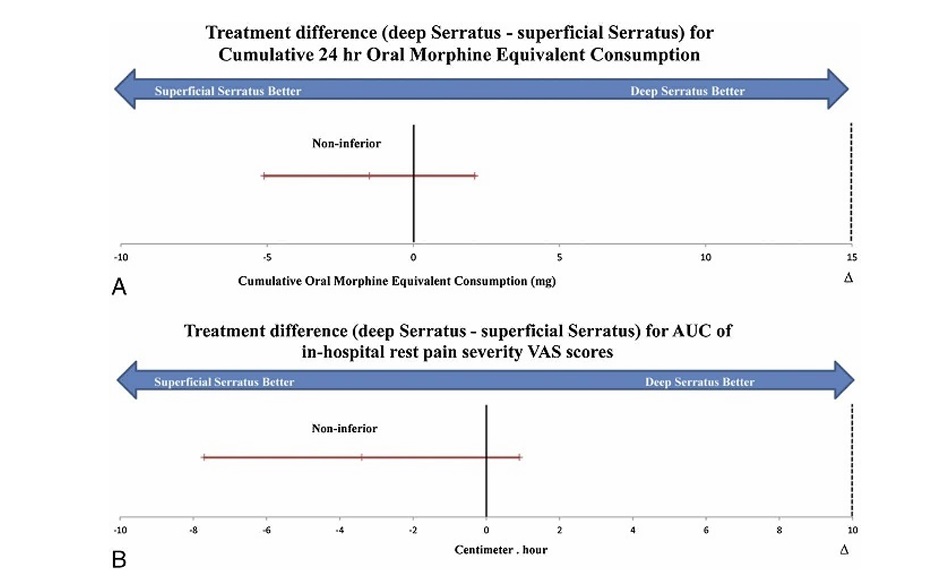
FIGURE 3. Noninferiority diagram with the observed differences between the deep serratus block group and the superficial serratus block group (deep serratus − superficial serratus) in the propensity-matched cohort for (A) cumulative in-hospital oral morphine equivalent consumption. B, Area under the curve of in-hospital postoperative rest pain severity VAS scores. The dashed line designates the noninferiority margin (Δ). The error bars designate the 95% CI of the difference. The diagram indicates noninferiority of deep serratus block for the 2 primary outcomes examined.
factors are not routinely collected. In addition, the role of local an esthetic infiltration, an effective analgesic modality,38 could not be examined, as it is rarely administered in conjunction with a serratus block at our institution. We recognize that our conclusions may not be necessarily generalizable to other fascial plane blocks, breast procedures,
patient populations, or local surgical practices, such as arm positioning and movement during surgery, which could theoretically influence local anesthetic spread within the axilla. Similarly, the patient population in our ambulatory center consists primarily of
healthy women (ASA classification I or II), with minimal comor bidities, which may not be the case in other institutions. Finally, our outcomes were restricted to early postoperative experience; we did not capture data regarding the analgesic effects of the
blocks beyond hospital discharge.
Based on this propensity-matched observational study, our results suggest that the deep serratus block technique is associated with early postoperative analgesia that isaseffective (within anacceptable margin) as the superficial serratus technique following ambulatory breast cancer surgery. The 2 blocks were also similar with respect to in-hospital opioid consumption and rest pain scores. These new findings are important to inform both current clinical practices and future prospective studies.
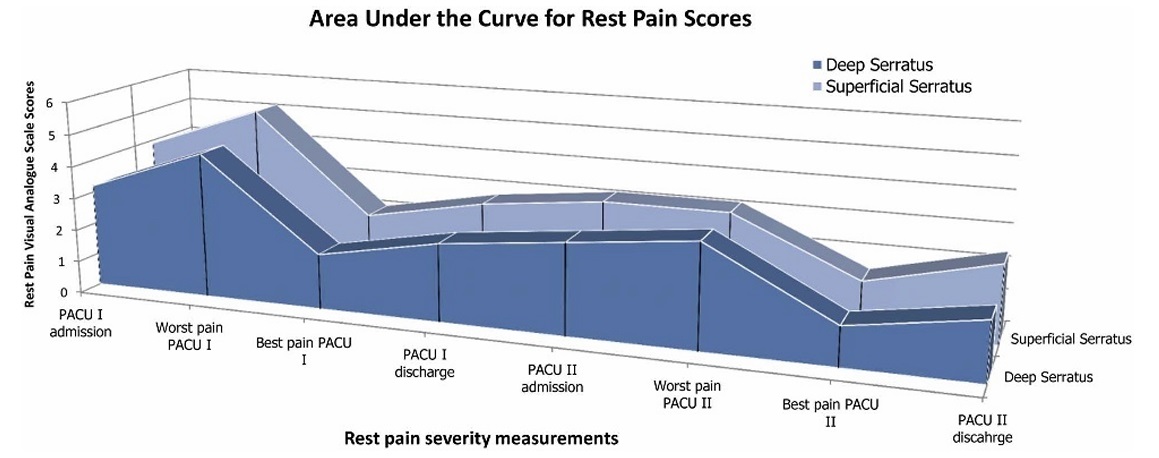
FIGURE 4. Plot of the AUC of the mean in-hospital postoperative rest pain severity VAS scores.
1. De la Torre PA, García PD, Alvarez SL, Miguel FJ, Pérez MF. A novel
ultrasound-guided block: a promising alternative for breast analgesia.
Aesthet Surg J. 2014;34:198–200.
2. Bhoi D, Pushparajan HK, Talawar P, Kumar A, Baidya DK. Serratus
anterior plane block for breast surgery in a morbidly obese patient. JClin
Anesth. 2015;33:500–501.
3. Bouzinac A, Brenier G, Dao M, Delbos A. Bilateral association of
Pecs I block and serratus plane block for postoperative analgesia after
double modified radical mastectomy. Minerva Anestesiol. 2015;81:
589–590.
4. Hards M, Harada A, Neville I, et al. The effect of serratus plane block
performed under direct vision on postoperative pain in breast surgery.
JClinAnesth. 2016;34:427–431.
5. Hetta DF, RezkKM.Pectoralis-serratus interfascial plane block vs thoracic
paravertebral block for unilateral radical mastectomy with axillary
evacuation. JClinAnesth.2016;34:91–97.
6. Abdallah FW, MacLean D, Madjdpour C, Cil T, Bhatia A, Brull R.
Pectoralis and serratus fascial plane blocks each provide early analgesic
benefits following ambulatory breast cancer surgery: a retrospective
propensity-matched cohort study. Anesth Analg. 2017;124:2008–2020.
7. Tighe SQ, Karmakar MK. Serratus plane block: do we need to learn
another technique for thoracic wall blockade? Anaesthesia.2013;68:
1103–1106.
8. BlancoR,ParrasT,McDonnellJG,Prats‐GalinoA.Serratus plane block: a
novel ultrasound‐guided thoracic wall nerve block. Anaesthesia.2013;68:
1107–1113.
9. Kettner SC. Dexmedetomidine as adjuvant for peripheral nerve blocks.
Br JAnaesth. 2013;111:123.
10. Khemka R, Chakraborty A, Ahmed R, Datta T, Agarwal S.
Ultrasound-guided serratus anterior plane block in breast reconstruction
surgery. A A Case Rep. 2016;6:280–282.
11. Ueshima H, Iwamoto W, Otake H. Serratus plane block for a contraction
of the latissimus dorsi muscle. Reg Anesth Pain Med.2016;41:
411–412.
12. Zocca JA, Chen GH, Puttanniah VG, Hung JC, Gulati A.
Ultrasound-guided serratus plane block for treatment of postmastectomy
pain syndromes in breast cancer patients: a case series. Pain Pract.2017;
17:141–146.
13. Khalil AE, Abdallah NM, Bashandy GM, Kaddah TA. Ultrasound guided
serratus anterior plane block versus thoracic epidural analgesia for
thoracotomy pain. J Cardiothorac Vasc Anesth. 2017;31:152–158.
14. Okmen K, Okmen BM, Uysal S. Serratus anterior plane (SAP) block
used for thoracotomy analgesia: a case report. Korean J Pain.2016;29:
189–192.
15. Piracha MM, Thorp SL, Puttanniah V, Gulati A. “A tale of two planes”:
deep versus superficial serratus plane block for postmastectomy pain
syndrome. Reg Anesth Pain Med. 2017;32:259–262.
16. Dzwierzynski WW. Complete lymph node dissection for regional nodal
metastasis. Clin Plast Surg. 2010;37:113–125.
17. Harter LP, Curtis JS, Ponto G, Craig PH. Malignant seeding of the needle
track during stereotaxic core needle breast biopsy. Radiology. 1992;185:
713–714.
18. Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by
large-gauge needle core biopsy of the breast? AJR Am J Roentgenol.1999;
173:1303–1313.
19. ChaoC,Torosian MH, BoraasMC, etal. Local recurrence of breast cancer
in the stereotactic core needle biopsy site: case reports and review of the
literature. Breast J.2001;7:124–127.
20. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting
of Observational Studies in Epidemiology (STROBE) statement: guidelines
for reporting observational studies. Prev Med.2007;45:247–251.
21. Merskey H, Bogduk N. International Association for the Study of Pain.
Task Force on Taxonomy. Classification of chronic pain: descriptions of
chronic pain syndromes and definitions of pain terms. Available at
https://www.iasp-pain.org/PublicationsNews/Content.aspx?
ItemNumber=1673.
22. Aldrete JA.Modifications tothe postanesthesia score for use in ambulatory
surgery. J Perianesth Nurs. 1998;13:148–155.
23. Rosenbaum PR, Rubin DB. The central role of the propensity score in
observational studies for causal effects. Biometrika.1983;70:41–55.
24. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its
persistence following breast cancer surgery. Pain. 2005;119:16–25.
25. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a
critical review of risk factors and strategies for prevention. J Pain.2011;12:
725–746.
26. Austin PC. Some methods of propensity‐score matching had superior
performance to others: results of an empirical investigation and Monte
Carlo simulations. Biom J. 2009;51:171–184.
27. Canadian Pharmacists Association. Compendium of Pharmaceuticals
and Specialties: The Canadian Drug Reference for Health Professionals.
45th ed. Ottawa, Ontario, Canada: Canadian Pharmacists
Association; 2010.
28. Dmitrienko A, Tamhane AC. Gatekeeping procedures with clinical trial
applications. Pharm Stat. 2007;6:171–180.
29. Mascha EJ, Turan A. Joint hypothesis testing and gatekeeping
procedures for studies with multiple endpoints. Anesth Analg. 2012;114:
1304–1317.
30. Todd KH, FunkKG,FunkJP, Bonacci R.Clinical significance of reported
changes in pain severity. Ann Emerg Med. 1996;27:485–489.
31. CepedaMS,AfricanoJM,PoloR,AlcalaR,CarrDB.Whatdeclineinpain
intensity is meaningful to patients with acute pain? Pain. 2003;105:
151–157.
32. Sakamoto H, Akita K, Sato T. An anatomical analysis of the relationships
between the intercostal nerves and the thoracic and abdominal muscles in
man.I. Ramification ofthe intercostal nerves. ActaAnat(Basel). 1996;156:
132–142.
33. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping
and sentinel lymphadenectomy for breast cancer. Ann Surg.1994;220:
391–398.
34. Macéa JR, Fregnani JH. Anatomy of the thoracic wall, axilla and breast.
Int J Morphol. 2006;24:691–704.
35. Jatoi I, Kaufmann M, Petit JY. Atlas of Breast Surgery.SpringerScience&
Business Media; Heidelberg, Germany, 2005.
36. Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity
score methods for estimating marginal odds ratios in case of small sample
size. BMC Med Res Methodol. 2012;12:70–80.
37. Peikes DN, Moreno L, Orzol SM. Propensity score matching: a note of
caution for evaluators of social programs. Am Stat. 2008;62:222–231.
38. Byager N, Hansen MS, Mathiesen O, Dahl JB. The analgesic effect of
wound infiltration with local anaesthetics after breast surgery: a qualitative
systematic review. Acta Anaesthesiol Scand. 2014;58:402–410.
