Background and Objectives: Nerve blockade of the lateral femoral cutaneous (LFC) nerve provides some analgesia after hip surgery. However, knowledge is lacking about the extent of the cutaneous area anesthetized by established LFC nerve block techniques, as well as the success rate of anesthetic coverage of various surgical incisions. Nerve block techniques that rely on ultra sonographic identification of the LFC nerve distal to the inguinal ligament can be technically challenging. Furthermore, the branching of the LFC nerve is variable, and it is unknown if proximal LFC nerve branches are anes the tized using the current techniques. The primary aim of this study was to investigate a novel ultrasound-guided LFC nerve block technique based on injection into the fat-filled flat tunnel (FFFT), which is a duplicature of the
fascia lata between the sartorius and the tensor fasciae latae muscle, in order to assess the success rate of anesthetizing the proximal LFC nerve branches and covering of the different surgical incisions used for hip surgery.
Methods: First, a cadaveric study was conducted in order to identify an FFFT injection technique that would provide adequate injectate spread to the proximal LFC nerve branches. Second, a clinical study was conducted in a group of 20 healthy volunteers over 2 consecutive days. On trial day 1, successful complete anesthesia of the LFC nerve was defined by performing a suprainguinal fascia iliaca block bilaterally in each subject. On trial day 2, a triple-blind randomized controlled trial compared the effect of the novel ultrasound-guided LFC nerve block technique for bupivacaine versus placebo. The primary end point was the success rate of anesthesia of the proximal cutaneous area innervated by the LFC nerve for the FFFT injection with bupivacaine versus placebo.
Results: Adequate spread of injectate to the proximal LFC nerve branches in cadavers was obtained by injecting 10 mL with dynamic needle-tip tracking in the FFFT. Application of this technique in the randomized controlled trial provided anesthesia of the lateral thigh with a success rate of 95% (95%
confidence interval, 73.9%–99.8%) for the active side and 0% for placebo (P < 0.001). The proximal branches were anesthetized with a success rate of 68% (95% confidence interval, 43.4%–87.4%) on the active side. The proximal extent of the anesthetized cutaneous area was on average 7.9 cm distal to the greater trochanter.
Conclusions: This novel LFC nerve block technique is easy and quick and reliably produces anesthesia of the lateral thigh. The greater trochanter is rarely included in the area of anesthesia, which reduces the coverage of each specific surgical incision. The success rate of 68% in anesthetizing the proximal nerve branches must be further evaluated by future research.
(RegAnesthPainMed2018;43: 357–366)
The lateral femoral cutaneous (LFC) nerve innervates the skin of the lateral thigh, and the effect of peripheral LFC nerve blockade on pain after hip surgery has been assessed in several studies.1–7 These studies are heterogeneous concerning surgical approach and combination of nerve blocks. However, across these differences, an analgesic effect of the LFC nerve block has been
reported in patients after hip surgery.
The success of the LFC nerve block in reducing postoperative pain relies on adequate anesthesia of the area of the surgical incision. On this matter, specific issues should be given attention. First, most surgical incisions for hip surgery involve the proximal part of the lateral thigh, hence the importance of anesthetizing the proximal LFC nerve branches. Moreover, the location of the surgical incision differs among the various hip surgery techniques, and each approach should be evaluated separately in relation to the area of cutaneous anesthesia. Finally, anatomical variations of the LFC nerve and its branching are frequent (Fig. 1). These variations make details of the regional anesthetic technique im portant because all techniques may not necessarily anesthetize all LFC nerve branches and may therefore anesthetize different skin areas. Studies that present and investigate specific techniques of the LFC nerve block are few and do not provide detailed infor mation on the area anesthetized by the applied techniques.11–15 Similarly, in-depth details of the applied surgical techniques are mostly absent from the few studies focusing on mapping the anes the tized skin area after LFC nerve blockade.16–18 Previously published ultrasound-guided nerve block techniques are targeting the LFC nerve, or one of its branches, deep to the in guinal ligament or anterior to the sartorius muscle. Anesthetizing the LFC nerve deep to the inguinal ligament holds the risk of con comitant femoral nerve block, whereas targeting the nerve anterior to the sartorius muscle is associated with the risk of variable success rates and technical challenges such as poor visualization of all the branches of the target nerve.11,13 Distal to the anterior superior iliac spine (ASIS), the LFC nerve is consistently found in a fat-filled flat tunnel (FFFT)19 formed by a double layer of the fascia lata between the sartorius and the tensor fasciae latae muscle.20–22 Ultrasonography easily identifies the FFFT and the LFC nerve therein, and the FFFT may serve as a target of injection to anesthetize the LFC nerve. The aim of this study was to assess the anesthetic effect of a novel ultrasound-guided LFC nerve block technique in the FFFT in a randomized controlled trial (RCT). Prior to the randomized
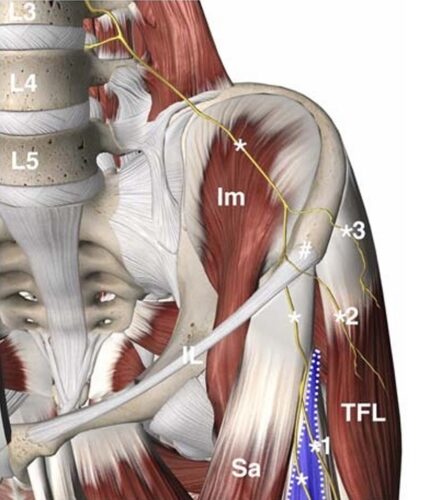
FIGURE 1. The common course of the LFC nerve asitruns deep to the iliac fascia on the ventral surface of the iliac muscle (Im) and descends toward the ASIS (#). It originates as an independent nerve fromtheL2–3spinal segments. The nerve passes deep to the inguinal ligament (IL) medial to the ASIS and crosses the superficial surface of the sartorius muscle (Sa). The nerve gives off 1 to 2 branches (1) to the proximal lateral thigh as it enters into the FFFT (blue area) between the sartorius muscle and the tensor fasciae latae muscle (TFL) muscle.8 Important variations of the common course of the nerve include an LFC nerve branch (22%–28%) entering the proximal thigh by crossing the ASIS (2) or less frequently a more posterior LFC branch (11%–18%) crossing the iliac crest posterior to the ASIS (3).9,10 The figure is a modified excerpt from Essential Anatomy 5, with permission from 3D4Medical (www.3d4medical.com).
trial, an initial explorative dissection study was conducted in order to identify an efficient technique of injection into the FFFT that would include also the proximal branches of the LFC nerve. In addition, bilateral supraing uinal fascia iliaca compartment (SI-FIC) blocks were carried out in all volunteers and served as a reference of complete LFC nerve blockade.23 Our objective was to compare the success rate of anesthesia of the proximal LFC nerve branches and coverage of the surgical incisions for hip surgery of active LFC nerve block in the FFFT to placebo in a triple-blind RCT. The hypothesis was that the success rate of proximal anesthesia of the lateral thigh after the LFC nerve block in the FFFT was sig nificantly higher with bupivacaine compared with placebo.
Study 1: Dissection Study of Spread of Injectate
Ten cadavers (7 female, 3 male), donated to the Division of Clinical and Functional Anatomy of the Medical University of Innsbruck for scientific and educational purposes,24,25 were dis sected to evaluate the spread of dye following 2 different techniques of injection. All cadavers were preserved using an arterial injection
of an ethanol-glycerol solution and immersion in phenolic acid in water for 1 to 3 months.26,27 Injections were carried out under real-time ultrasound guidance, using an Esaote ultrasound system with a high-frequency 13–4MHz linear array probe (AL2442 probe, Esaote MyLab Seven US System; Esaote, Genoa, Italy). The LFC nerve was identified in the FFFT in the transverse cross-sectional view approximately 10 cm distal
to the ASIS, between the sartorius and the tensor fasciae latae muscles. The LFC nerve was traced proximally with ultra son og raphy to its point of entry into the FFFTafter crossing the anterior surface of the sartorius muscle. A 70-mm, 22-gauge, short-bevel needle (Stimuplex D Plus; B.Braun, Melsungen, Germany) was inserted out-of plane into the FFFT (Fig. 2), positioning the needle tip next to the nerve (Fig. 3). After randomization (right or left side), methylene blue 2 % was injected bilaterally using 2 different techniques of injection: On 1side, 5 mL was injected into the FFFT, keeping the needle tip immobile. On the contralateral side, 10 mL was injected intermittently into the FFFTand simultaneously advancing the needle tip inside the FFFT toward the ASIS, while tracking the needle tip dynamically with ultrasonography in real time (ie, dynamic
needle-tip tracking).
If the FFFT was multicompartmentalized, the subdivision
containing the LFC nerve branch that could be traced most proximally was chosen as the point of injection.
After completion of each injection, the investigator who performed the injection, left the dissection room, and another investigator (B.M.), who was blinded to the randomization and not present during the injections, carried out the dissection and documented the spread of dye. Dissections were performed bilaterally in all 10 specimens. In order to decrease the risk of artifactual spread of dye, each dissection was performed immediately after injection. Accordingly, 2 initial incisions, the first starting 2 fingerbreadths cranioposterior to the ASIS and extending horizontally 10 cm medially, and a second sagittal incision of approximately 20 cm over the proximal-lateral aspect of thigh went through the skin and subcutaneous tissue overlying the muscles. Subsequently, the triangular tissue flap was raised and reflected medially to expose the inguinal region and the fascia lata of the proximal thigh.
The latter was split along the inguinal ligament, as well as distally over the tensor fasciae latae muscle. This exposed the sartorius and tensor fasciae latae muscles as well as the FFFT between
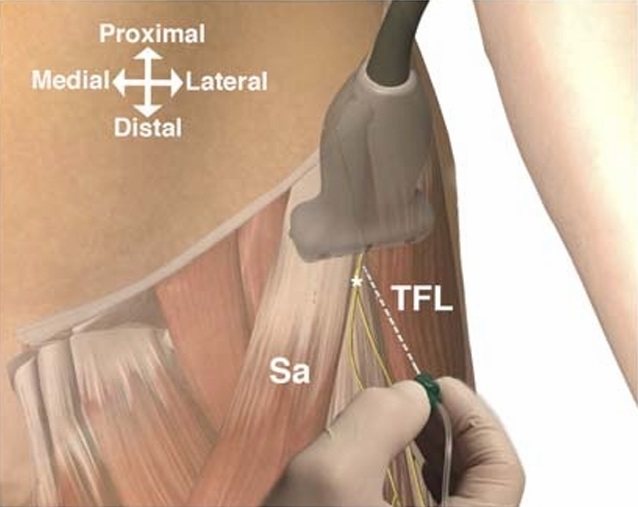
FIGURE 2. Scanning in the transverse plane provides the easiest recognizable ultrasonographic image of the FFFT and the LFC nerve. The out-of-plane needle insertion provides the shortest needle trajectory and is warranted for dynamic ultrasonographic needle-tip tracking in order to facilitate proximal spread during injection. Sa indicates sartorius muscle; TFL, tensor fasciae latae muscle; asterisk (*), LFC nerve. The figure is a modified excerpt from Essential Anatomy 5, with permission from 3D 4 Medical (www.3d4medical.com).
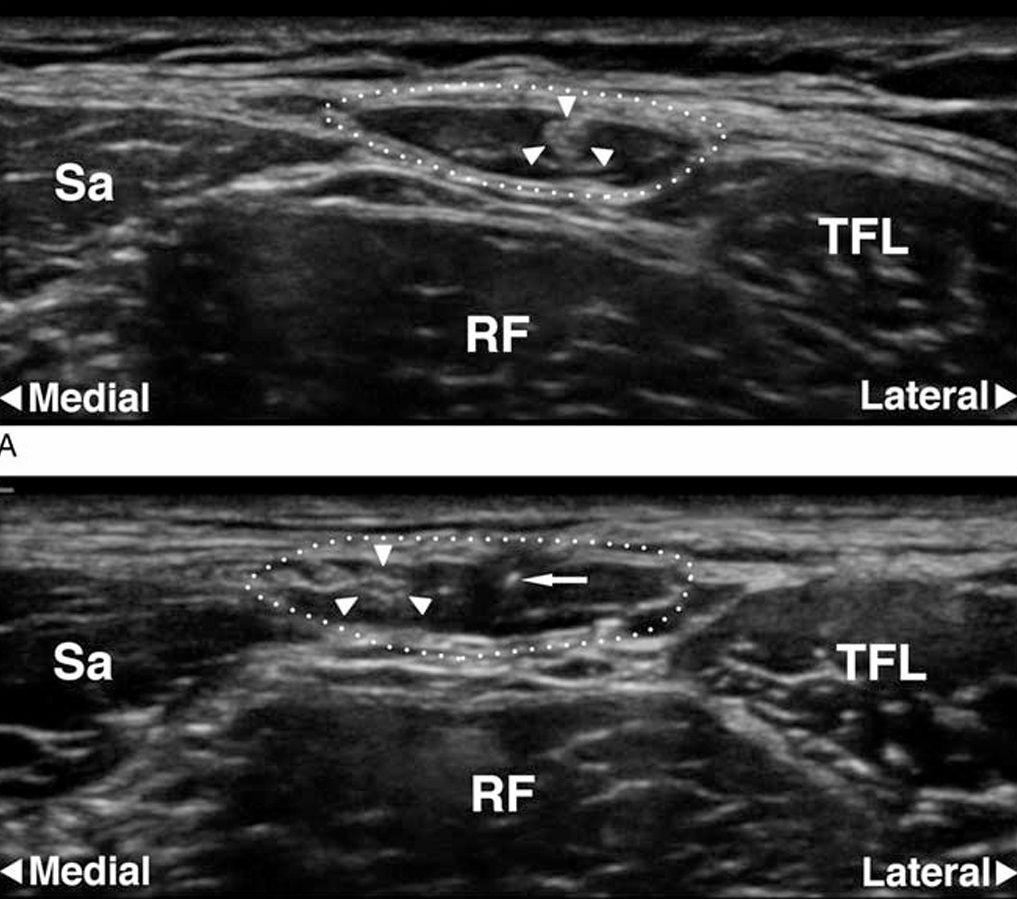
FIGURE 3. A and B, Ultrasonographic images of the FFFT. A, Transverse scan 10 cm distal to the ASIS. The dotted line indicates the circumference of the FFFT. The hyperechoic LFC nerve (arrowheads) is surrounded by the anechoic (ie, black) fat inside the FFFT. B, Transverse scan 5 cm distal to the ASIS.The needletip(arrow)is visualized incross section. The LFC nerve (arrowheads) has just entered into the FFFT after crossing the anterior surface of the sartorius muscle (Sa). RF indicates rectus femoris muscle; TFL, tensor fasciae latae muscle.
them. Finally, all branches of the LFC nerve in the exposed area were identified and followed. Dissection was not extended posterior to the ASIS to identify possible LFC nerve branches crossing the iliac crest at this position. The end points of the dissection study were success of coloring all branches of the LFC nerve and success of spread proximally and anterior to the sartorius muscle using the 2 different techniques described above. The best injection technique as identified in the dissection study would be applied in the planned randomized con trolled comparison of active LFC nerve block versus saline in the clinical study.
Study 2: Clinical Study of the Regional
Anesthetic Effect
The study was registered in the EudraCT database (#2016 003213-98, August 17, 2016) and was approved by the Danish Health and Medicines Authority, the Central Denmark Region Committee on Biomedical Research Ethics, and the Danish Data
Protection Agency and was monitored by the Good Clinical Practice Unit at Aalborg and Aarhus University Hospitals. Twenty ASA I and II (American Society of Anesthesiology
Physical Status classification) subjects 18 years or older were recruited through a Danish Web site for research volunteers. Subjects who were non-Danish or non-English speakers; had aweight of less than 55 kg, infection, or sensory neurological disorders in the area of the hip; and were pregnant or allergic to local anesthetic were excluded.
The study was conducted as a triple-blind RCTof bupivacaine versus placebo for the LFC nerve block on trial day 2 after prior bilateral assessment of the area of complete anesthesia of the LFC nerve after the SI-FIC block on trial day 1 (Fig. 4). The study was carried out at the Department of Day Surgery, Aarhus University Hospital, Denmark, during a 2-day session in December 2016. Written informed consent was obtained from all subjects prior to the start of the trial. The volunteers received payment for participation.
The effect of blinded injection with either local anesthetic or placebo in the FFFTwas compared with the anesthetized cutaneous area of the suprainguinal fascia iliaca compartment block, which served as a reference for complete anesthesia of the LFC in its intrapelvic trajectory prior to any branching of the LFC nerve. The investigators who performed the nerve blocks also recorded the data concerning the nerve block procedure. Independent investigators recorded all other end points and performed all
other procedures. Each step in the trial procedure was carried out in separate rooms by individual investigators blinded to the assessments of all other investigators.
Trial Day 1: Bilateral Assessment of Maximum Area
of Anesthesia
On the first day, the trial was conducted in 8 (1–8) steps in order to perform bilateral assessment of the maximum area of an esthesia after blockade of the LFC nerve using the SI-FIC block:
(1) Surgical incision lines as well as other reference lines were marked bilaterally on the skin with a UV pen (364 nm; J & Maya, Højbjerg, Denmark) (Figs. 5A, B). An orthopedic surgeon marked the surgical incision lines (J.B.), which are
FIGURE 4. Flow diagram of the volunteer trial.
approaches for hip surgery.28,29
(2) Prior to nerve blockade, bilateral baseline knee extensor muscle strength was recorded as the average of 3 measurements of maximum voluntary isometric contraction (MVIC), using a handheld dynamometer (Commander Muscle Tester; JTECH Medical, Midvale, Utah).30
(3) Bilateral SI-FIC nerve blocks were then performed in or der to anesthetize the LFC nerve in its intrapelvic trajectory prior to any LFC nerve branching. Heart rate and oxygen sat uration were monitored continuously during the block procedure. An 80-mm, 22-gauge, short-bevel needle (Ultraplex; B.Braun) was inserted in-plane distal to the inguinal ligament guided by ultrasound (15–6 MHz linear transducer, SonoSite X-Porte;
FUJIFILM SonoSite, Inc, Bothell, Washington). The fasciailiaca was penetrated, and the fascial plane between the iliac muscle and the fascia iliaca was hydrodissected by the injectate while advancing the needle tip intrapelvically.23 Twenty milliliters of 1% lidocaine with 1:200,000 epinephrine was injected bi laterally (Lidokain-Adrenalin SAD; Amgros I/S, Copenhagen, Denmark).
(4) One hour after completion of each pair of bilateral SI-FIC nerve blocks, postblock measurements of the knee extensor muscle strength were recorded bilaterally.
(5) Subsequently, the anesthetized area was defined using stan dardized pinprick (Neuropen; Owen Mumford, Woodstock, United Kingdom). AUV pen was used tomark points (invisible to the naked eye) along the margin of the area that was identified by pinprick.
(6) Another investigator illuminated the areas with UV light bilaterally and drew straight connecting lines with a UV pen between the marked points bilaterally and collected end
point data.
(7) To assess interobserver variations, definition of the proximal margin of the anesthetized area was repeated with pinprick bilaterally and marked with a UV pen.
(8) The distance between the duplicate registrations was measured on the straight line intersecting the greater trochanter and 360 the lateral edge of the patella (GT-P line) after illuminating the area with UV light.
Trial Day 2: Randomized Controlled Trial Procedure On the second day, the trial was conducted in 5 (9–13) steps in order to perform a randomized controlled comparison of
bupivacaine versus placebo for the LFC nerve block:
(9) Two independent investigators, responsible only for the trial medicine, sealed 20 consecutively numbered envelopes, each with 1 syringe containing 10 mL 0.25% bupivacaine (Marcain; AstraZeneca A/S, Albertslund, Denmark) and 1 syringe contain
ing10mLofisotonicsaline.Eachsyringeineverypairwasran domlymarkedeither“left” or “right.” The investigators kept the randomization list until after the data analysis was completed.
(10) The skin of the lateral thigh was assessed bilaterally with pinprick in each subject to ensure complete cessation of anes the sia from trial day 1. The subjects were then randomized to a number between 1 and 20. This number decided which enve
lope containing the trial medicine should be used for the following LFC nerve block.
(11) Lateral femoral cutaneous nerve block technique: The LFC nerve block was performed bilaterally with the subject in the supine position. The FFFT injection technique was identical to the most efficient of the 2 ultrasound-guided technique described in METHODS of the dissection study, using 10 mL of injectate and an echogenic nerve block needle (Ultraplex; B.Braun). A linear high-frequency transducer (15–6 MHz linear transducer, SonoSite X-Porte; FUJIFILM SonoSite, Inc) was used to dy namically guide the proximal advancement of the needle tip during injection.
(12) Thirty minutes after completion of the LFC nerve block, the anesthetized area was assessed with pinprick. This proce dure was performed as described above except that the marking of the points was done with a normal pen. Two investigators assessed the 2 sides independently in separate rooms. If no area
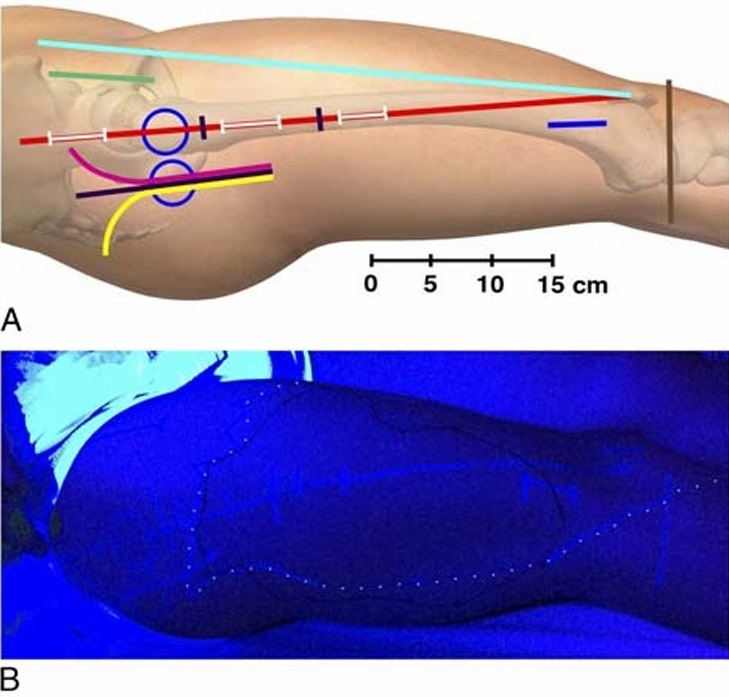
FIGURE 5. A,Graphical depiction of the reference and incision lines marked at the beginning of the trial with a UV marker. The anterior, anterolateral, direct lateral, and posterior approaches are used for hemiarthroplasty and total hip arthroplasty. Sliding hip screw devices, IM nails, and parallel implants (screws and hook-pins) are used for femoral neck and extracapsular proximal femoral fractures. Anterior blue circle, surface projection of the greater trochanter with the subject in the supine position and 20-degree internal rotation; posterior blue circle, surface projection of the greater trochanter with the subject in the lateral position; red line, GT-P line (straight line intersecting the greater trochanter and the lateral edge of the patella); cyan line, ASIS-P line (straight line intersecting the ASIS and the lateral edge of patella); brown line, tibiofemoral joint line; green line, anterior incision; magenta line, anterolateral incision; black line, direct lateral incision; yellow line, posterior incision; proximal white line on the red line, incision for entry point of IM nail; intermediate white line on the red line, incision for proximal locking of an IM nail; distal white line on the red line, incision for distal locking screws of a short IM nail; blue line, incision for distal locking screws of a long IM nail; the red line segment between the 2 black markings, incision for sliding hip screw or parallel implants (parallel screws or hook-pins). The figure is a modified excerpt from Essential Anatomy 5, with permission from 3D 4 Medical (www.3d4medical.com).
B, Photography of the lateral thigh while illuminated with UV light by the last investigator on day 2 of the clinical trial. Incision and reference lines are the same as in A. The solid black line outlines the area anesthetized by the LFC nerve block. The UV marking of the area anesthetized by the SI-FIC nerve block on day 1 is marked with a superimposed white dotted line.
of anesthesia was identified, a sham marking of the skin was carried out in order to blind subsequent investigators.
(13) Following the assessment, the right and left sides were illminated with UV light (Fig. 5B), each side separately in separate rooms by different investigators, in order to record the anesthesia outcome data.
Primary End Point
Theprimaryend point wassuccessful proximal anesthesia of the lateral thigh after the LFC nerve block of bupivacaine versus placebo LFC nerve block in the FFFT in the RCT. Successful proximal anesthesia of the lateral thigh was defined as anesthesia
extending to the proximal border of the area anesthetized with the SI-FIC block performed on trial day 1, which served as the reference for complete anesthesia of the LFC nerve. A maximum deviation of 3 cm was allowed (measurement error) based on previous pilot data. The distance was measured on the axis of the line intersecting the greater trochanter and the lateral edge of the patella (GT-P line).
Secondary End Points
The study included the following 12 (a-m) secondary out comes: (a) distance from the proximal margin of the anesthetized area to the greater trochanter after the LFC nerve block; (b) distance from the proximal margin of the anesthetized area to the greater
trochanter after the SI-FIC block; (c) distance from the distal margin of the anesthetized area to the tibiofemoral joint line after the LFC nerve block; (d) distance from the distal margin of the anes the tized area to the tibiofemoral joint line after the SI-FIC block (the distal extent of the SI-FIC block was not measured beyond the tibiofemoral joint line; in case of anesthesia distal to the joint line, the distance was registered as 0 cm); (e) inclusion of each marked surgical incision line in the anesthetized area after the LFC nerve block (total, partial, or absent inclusion); ( f )success rate of total inclusion of the distal locking incision of the long intramedullary (IM) nail; (g) anteromedial and posterolateral extent of the anesthetized area after the LFC nerve block; (h)fre quency of motor block of the femoral nerve after the LFC nerve block (motor block was defined as at least 25% reduction in MVIC compared with baseline measurements); (i) frequencyof motor block of the femoral nerve after the SI-FIC block; ( j) duration of anesthesia after the LFC nerve block; (k) quality of ultraso nographic visualization of the LFC nerve inside the FFFT (good, reduced, poor); (l) LFC nerve block performance time; (m) discomfort during LFC nerve block performance (numeric rating scale [NRS] 0–10 from no discomfort [0] to worst imaginable discomfort [10]). Data were collected and recorded using the secure, online database system REDCap (6.15.4 Vanderbilt University, Nashville, Tennessee).
Statistical Analysis
Statistics were analyzed using Stata 13.1 (StataCorp LLC, College Station, Texas). Differences between paired categorical variables (success/failure) were analyzed with Mc Nemar test and presented with rate, P value, and approximated confidence intervals
(CIs). The level of significance was 0.05. Other results are presented as mean with 95% CI and SD, or median and interquartile range as appropriate. Descriptive bilateral variables (distances, procedure duration, discomfort) were analyzed using data from the
sides randomized to active treatment on day 2 only. Our null hypothesis was no difference in success rate for local anesthetic compared with placebo regarding anesthesia of the proximal branches of the LFC nerve using the novel LFC nerve block technique (see Primary End Point).
Dissection Study
The LFCnerve and all its branches were stained in all 10 cadaversides injected with 10mLusingdynamicneedle-tiptracking
(100%; 97.5% CI, 69.2%–100%[1-sided]). In 2 of the 10 cadaver
sides injected with 5 mL and immobile needle tip, the most prox
imal of the LFC nerve branches was not stained (Fig. 6). In the
other 8 cadaver sides injected with 5 mL, all the branches were
stained (80%; 95% CI, 44.4%–97.5%), and the difference of
20% (95% CI, −14.8% to 54.8%) between the 2 techniques was
not statistically significant (P =0.5).
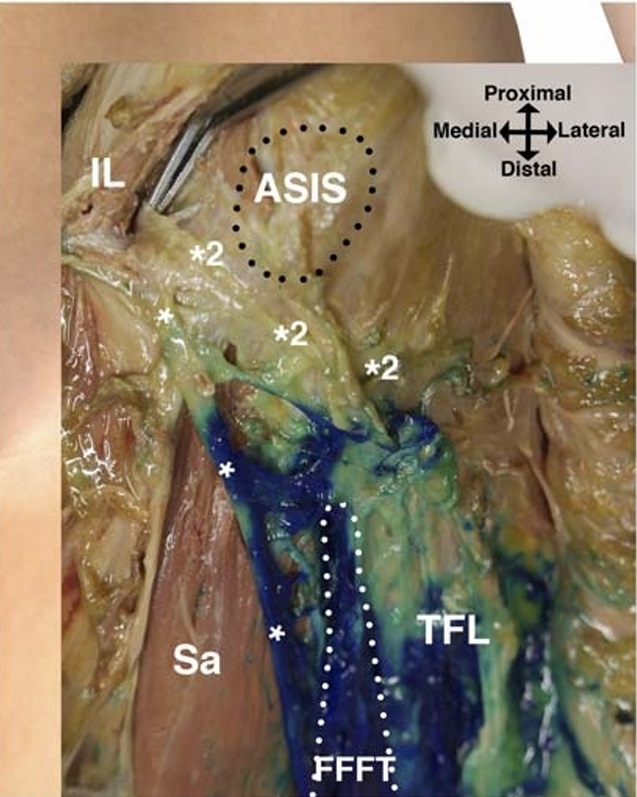
FIGURE 6. Dissection of the left side of a cadaver in the supine
position after injection of 5 mL of methylene blue into the FFFT
(white dotted line). Most of the dye is confined inside the FFFT with
some spread across the surface of the tensor fasciae latae muscle
(TFL). Across the surface of the sartorius muscle (Sa), the dye spread
only narrowly along the LFC nerve (*)towardtheinguinal
ligament (IL). In this cadaver, the proximal spread of dye did not
extend to the proximal LFC nerve branch (*2) branching off just
distal to the IL. The proximal LFCnervebranchcrossesjustinferiorto
the ASIS and ramifies to innervate the gluteal skin without
entering the FFFT.
The FFFTandtheLFCnerveinside the FFFTcouldbeiden
tified ultrasonographically in all cadaver sides. The mean distance
from the ASIS to the point of needle insertion was 6.9 cm (95%
CI, 5.5–8.2 cm) on the side injected with 5 mL and 5.2 (SD,
1.0) cm (95% CI, 4.5–5.9 cm) on the side injected with 10 mL.
The dye spread outside the FFFT, across the anterior surface of
the sartorius muscle deep to the fascia lata, in all 10 cases of the
cadaver sides injected with 10 mL, 100% (97.5% CI, 69.2%–100%
[1-sided]), and in 4 of the 10 cadaver sides injected with 5 mL,
40% (95% CI, 12.2%–73.8%). The difference of 60% (95% CI,
19.6%–100%) was statistically significant (P = 0.03).
Clinical Study
Twenty volunteers were enrolled in the study. One volunteer
did not show uponthe first day of the trial and was excluded. The
19 remaining volunteers (Table 1) all had successful bilateral
SI-FIC blocks on day 1. On trial day 2, after the randomized
bilateral LFC nerve blockade with bupivacaine versus placebo,
cutaneous anesthesia was found on the lateral thigh in 18 of the
19 active sides, 94.7% (95% CI, 73.9%–99.8%), and in 0 of 19
in the placebo sides, 0% (95% CI, 0.0%–17.6%), difference of
94.7% (95% CI, 79.4%–100%; P <0.001).
Primary Outcome
The LFC nerve block successfully produced anesthesia that
reached as proximal as the area anesthetized by the reference
SI-FIC block in 13 of the 19 active sides, 68.4% (95% CI,
43.4%–87.4%), and in 0 of the 19 placebo sides, 0% (95% CI,
0.0%–17.6%). The difference between active and placebo LFC
nerve block in the FFFT was 68.4% (95% CI, 42.3%–94.6%),
which is statistically significant (P < 0.001).
The success rates of the LFC nerve block to include the dif
ferent incisions in the anesthetized area are presented in Figure 7.
Total inclusion of the distal incision line for the long IM nail
locking screws demonstrated a success rate of 89.5% (95% CI,
66.9%–98.7%) after the SI-FIC nerve block.
Theproximalborderof theanesthetizedareawasdistal to the
greater trochanter on average after the LFC nerve block as well as
the reference SI-FIC nerve block. The mean distances from the
proximal margin of the greater trochanter to the proximal margin
of the area anesthetized by the LFC nerve block (Fig. 8) and the
SI-FIC block were 7.9 (SD, 8.0) cm (95% CI, 3.9–11.9 cm) and
6.8 (SD, 5.3) cm (95% CI, 4.2–9.3 cm), respectively.
Thedistal border of the anesthetized area after the LFC nerve
block was on average 7.6 (SD, 6.6) cm (95% CI, 4.3–10.9 cm)
proximal to the tibiofemoral joint line (Fig. 8).
The SI-FIC block produced an area of cutaneous anesthesia
of the lateral thigh that extended distal to the tibiofemoral joint
line in the majority of subjects, crossing the crus obliquely distal
to the patella to continue medially corresponding to the territory
of innervation of the saphenous nerve. As the distance to the joint
line was registered as 0 cm per protocol if the anesthesia extended
distal to the joint line, the mean distance from the area anes
thetized by the SI-FIC block to the tibiofemoral joint line was
0.7 (SD, 1.3) cm (95% CI, 0.1–1.3 cm).
Anteromedially, the anesthetized area after the LFC nerve block
crossed the anterior line connecting the ASIS to the lateral edge of the
patella (ASIS-P line) in 77.8% (95% CI, 52.4%–93.6%), with a mean
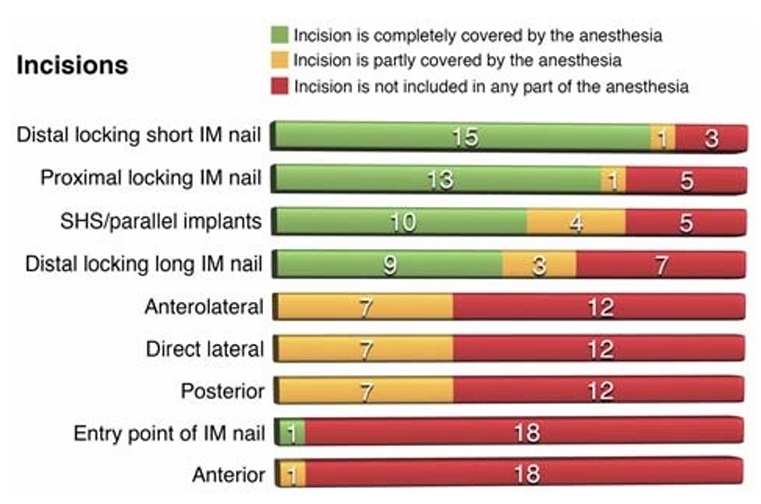
FIGURE 7. Success of the anesthetized area to cover specific
incisions after the FFFT nerve block. For each of the incisions, the
corresponding bar shows the actual number of successes, partial
successes, or failures.
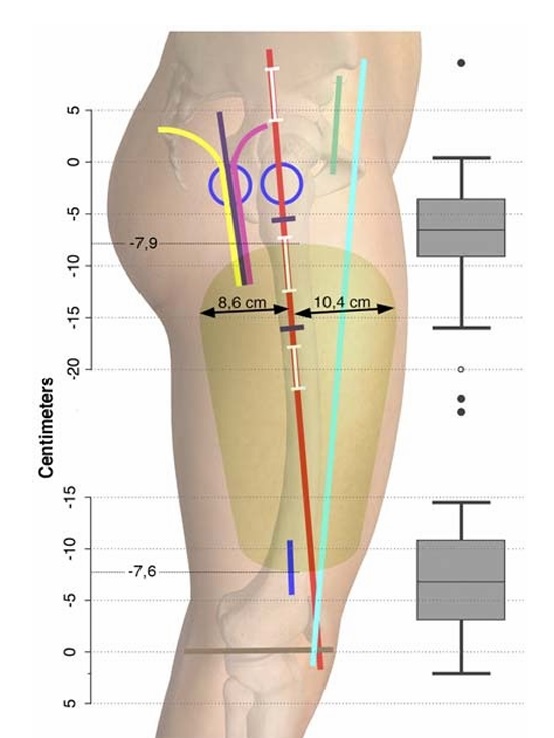
FIGURE 8. The green area summarizes the average extent of the
anesthetized area after an FFFT nerve block. The mean distances
defining the proximal-distal extent of the anesthetized area are as
follows: −7.9 cm below the greater trochanter and −7.6cmaboveto
the tibiofemoral joint line, respectively. The anteromedial-posterolateral
extension (double-headed arrows) was 10.4 cm to the GT-Pline from
the anteromedial margin and 8.6 cm from the posterolateral margin.
The box plots display the median, range, and interquartile range of
the distal and proximal distances from the anesthetized area to the
greater trochanter and the tibiofemoral joint line, respectively. Four
outliers are shown as 1 hollow circle belonging to the distal box plot
and the 3 solid circles belonging to the proximal box plot. Lines and
incisions are explained in Fig. 4A. The figure is a modified excerpt
from Essential Anatomy 5, with permission from 3D4Medical
(www.3d4medical.com).
distance to the line of 2.9 (SD, 3.6) cm (95% CI, −1.6 to 4.7 cm). Pos
itive values mean that the medial border of the anesthetized area is
medial to the ASIS-P line. The maximum anteromedial extent of
the anesthetized area, measured from the GT-P line and perpen
dicular to this line, was on average 10.4 (SD, 3.4) cm (95% CI,
8.7–12.1 cm), and the average maximum posterolateral extent
was 8.6 (SD, 2.4) cm (95% CI, 7.4–9.8 cm) (Fig. 8).
TheLFCnerveblockwassuccessfulinavoidingmotorblock
of the femoral nerve in 18 of the 19 subjects, 94.7% (95% CI,
74.0%–99.9%).Inthe1remainingsubject,thereductioninMVIC
was 33%. As a comparison, a motor block was avoided in only 2
of the 19 subjects, 10.5% (95% CI, 1.3%–33.1%), after receiving
the SI-FIC nerve block. The difference between the SI-FIC
and the LFC nerve block was 84.2%, which is statistically
significant (P <0.001).
The quality of the ultrasonographic visualization of the LFC
nerve inside the FFFTwas graded as good bilaterally in 16 of the
19 subjects. In 3 subjects, it was good on one side and reduced on
the other. The ultrasonographic visualization was not graded as
poor in any of the subjects. The duration of the LFC nerve block procedure was on average
3.4 (SD, 1.6) minutes (95% CI, 2.6–4.2 minutes) from the beginning
of the scan to the end of injection.
Discomfort during the LFC nerve block procedure was
scored using the NRSfrom0to10.MedianNRSwas3(interquar
tile range, 2–3).
Mean duration of anesthesia after the LFC nerve block,
basedonself-reporting,was27.0 hours(95%CI,20.4–33.6hours;
range, 10.2–63.7 hours).
The distance between the duplicate measurements of the
proximal margin of the anesthetized reference area on trial day 1
showed a mean of 1.2 (SD, 4.5) cm (95% CI, −1.0to3.3 cm; fifth
percentile, −7.5 cm).
Dissection Study
Prior to the present volunteer trial, we conducted a dissection
study to assess and compare the feasibility of novel LFC nerve
block techniques using injection into the FFFT.
The consistent location of the LFC nerve within the FFFT
had already been established.20–22 However, it was unknown if
proximal branches crossing the sartorius muscle distal to the ASIS
traverse the FFFT to reach the gluteal area or bypass the FFFT by
superficial or proximal trajectories. In case of all nerve branches
passing through the FFFT, a minimal volume injected into the
FFFT could be speculated to suffice. In case of nerve branches
bypassing the FFFT, concomitant spread of injectate outside the
FFFTwouldberequiredforcompleteanesthesiaofthe LFCnerve.
Hence, 2different techniques were compared in the dissection study
in order to obtain an intra-FFFT spread versus an extra-FFFT
spread: a low volume (5 mL) injected with a static needle-tip position
versus a larger volume (10 mL) injected with dynamic needle-tip
tracking. The injection of 10 mL of dye with simultaneous proximal
advancement of the needle caused the dye to spread proximally
throughout the FFFT and outside the FFFT, deep to the fascia lata
on the anterior surface of the sartorius muscle. An interesting finding
was that the dye spread tightly along the nerves beyond the overall
spread of the dye (Fig. 6), typically reaching the inguinal ligament
in the cadaver sides injected with 10 mL.
Injection into the FFFT facilitated spread in the correct layer
deep to the fascia lata when dye was observed outside the FFFT.
Spread of dye outside the FFFTand along the LFC nerve was of
consequence, because proximal LFC nerve branches did not always
enter the FFFT (Fig. 6) and were colored only by spread outside the
FFFT in some sides. The 2 proximal nerves unstained by the two
5-mL injections traversed the area just distal to the ASIS without
entering the FFFT.
Because of the more extensive spread with the 10-mL tech
nique with complete coloring of the proximal LFC nerve branches,
we selected this technique for the clinical study, although a statisti
cally significant difference of success in coloring all nerve branches
could not be demonstrated within this sample of 20 cadaver sides.
Certain limitations to the dissection study should be men
tioned. The dissection study was exploratory and not powered to
show a statistically significant difference in success rate of color
ing all nerve branches between the 2 FFFT injection techniques.
Furthermore, the spread of injectate found by dissection in soft
embalmed cadavers may not accurately reflect the spread of local
anesthetic in the living person.
It can be speculated that the observed nonsignificant differ
ence in distance between the ASIS and the point of needle inser
tion might have affected the results. In the case of the 2
injections that failed to color the proximal LFC nerve branches, both failures were caused by 5-mL injections not spreading out
side the FFFT. Probably the successful spread outside the FFFT
is primarily due to the volume of the injectate. Thus, it is unlikely
that a needle insertion point of the 5-mL injections a few centime
ters more proximal would have changed the results.
Finally, we explored only the spread of 5 and 10 mL of
injectate. Despite the high success rate of coloring all nerve
branches in the soft embalmed cadavers, it can be speculated that
a larger volume (eg, 15 mL)wouldbeneededtoachieve an equiv
alent spread in living patients. Consequently, assessment of the
optimal technique and volume of an LFC nerve block inthe FFFT
requires further research.
Clinical Trial
The clinical study of 19 humanvolunteers demonstrated that
10 mL of local anesthetic injected into the FFFTwhile advancing
the needle proximally with dynamic needle-tip tracking is suc
cessful in anesthetizing the LFC nerve with an approximate suc
cess rate of 95%. The reason why 1 block failed to anesthetize
the LFC nerve despite good ultrasonographic visualization of
the FFFTand the nerve therein can only be speculative. The needle
was inserted at the level of the anterior LFC nerve as it entered the
FFFT, followed by proximal dynamic advancement of the needle
tip in the FFFTwhile performing intermittent injections. This tech
nique is meant to ensure an initial spread around the anterior LFC
nerve main trunk, as well as a final proximal needle-tip position that
ensures spread to the proximal LFC nerve branches. It is possible in
the case of the single failure that injection was initiated too proximal
in the FFFT to reach the anterior LFC nerve main trunk.
The nerve block procedure was uncomplicated in terms of
easy ultrasonographic identification of the FFFT and LFC nerve,
short procedure time, and low procedural discomfort. Concomi
tant femoral motor block defined by more than a 25% reduction
in MVIC was avoided in all but 1 subject. The MVIC reduction
of 33%inthissubject, however, did not result in any subjective re
duction in muscle strength, and the subject was able to walk at
all times.
Theduration of anesthesiawas 27 hours on average, whichis
longer than expected with bupivacaine.31 Data on duration of LCF
nerve blockade are not available from other studies. It should be
noted that the duration was self-reported by each subject as the
time until the return of normal sensation in the anesthetized area
after they had returned home.
Theanesthetized area after the LFC nerve blockade extended
distal to the tibiofemoral joint line in only 1 subject, and the mean
distance was 7.6 cm proximal from the anesthetized area to the
joint line. After the reference SI-FIC block, the anesthetized area
of the lateral thigh extended distal to the tibiofemoral joint line
in 13 of the 19 subjects. In the remaining 6 subjects, the anesthe
tized area of the lateral thigh abutted the joint line without extend
ing beyond it. The difference in distal extent between the SI-FIC
and the LFC nerve block was expected because of blockade of
the anterior cutaneous branches of the femoral nerve with the
SI-FIC block. The distal extension of the anesthetized area is clin
ically relevant for anesthesia of the surgical incision for the distal
locking screws applied for the long IM nail, which is why the suc
cess of the SI-FIC block to cover this specific incision was included
as a specific secondary end point. Complete coverage of this partic
ular surgical incision line was raised from a success rate of 50% for
the LFC nerve block to 89.5% for the SI-FIC block. To the best of
our knowledge, a selective nerve block of the anterior cutaneous
branches of the femoral nerve has never been published.
The proximal border of the anesthetized area reached the
proximal margin of the greater trochanter in only 2 of 19 subjects after the LFC nerve block, as well as after the reference SI-FIC
block. On average, the proximal border of the anesthetized area
after both nerve block techniques was several centimeters distal
to the proximal margin of the greater trochanter. This result
strongly indicates that blockade of the LFC nerve, regardless of
nerve block technique, cannot be expected to have any analgesic
effect proximal to the greater trochanter. This is consistent with
the findings by Davies et al,17 who found only very limited anes
thesia of the surgical incisions used for hip arthroplasty after a
nerve block of the LFC nerve.
The surgical incision lines that we have included in this trial
represent the incision lines most commonly used for hip sur
gery.28,29 The assessment of anesthesia of each surgical incision
line suggests that the LFC nerve block does not anesthetize the
surgical incision of the anterior approach or the surgical incision
for the entry point of the IM nail. Furthermore, the anterolateral,
the direct lateral, and the posterolateral incision lines were only
partially included in the anesthetized area, suggesting that the
LFC nerve block is required but not sufficient to anesthetize the
area intersected by these surgical incisional approaches. Finally,
the incision lines representing the sliding hip screw and parallel
implants (parallel screws or hook pins), together with the proximal
IM nail locking and the short IM nail distal locking screws, were
all completely included in the anesthetized area in more than 50%
of the subjects. For these incision lines, the LFC nerve block is the
most relevant nerve block.
The primary end point of the present volunteer trial was suc
cess rate of cutaneous anesthesia of the area innervated by the
most proximal branches of the LFC nerveinthe randomizedcom
parison of bupivacaine versus placebo for the LFC nerve block.
The maximum proximal extent of this area was defined prior to
the RCTbythearea anesthetized with the reference SI-FIC block.
Accordingtothis definition, the LFC nerve block technique failed
to anesthetize the proximal branches of the LFC nerve in 32%
(Fig. 9). Thus, a success rate of 68% was recorded. This result
was statistically significant when compared with placebo. A
priori, a success rate of 100% was not anticipated because of
well-documented anatomical variations of the branching and
distribution patterns of the LFC nerve. In 22% to 28% of cadaver
sides in previous dissection studies, an LFC nerve branch is
reaching the proximal aspect of the thigh by crossing the ASIS,
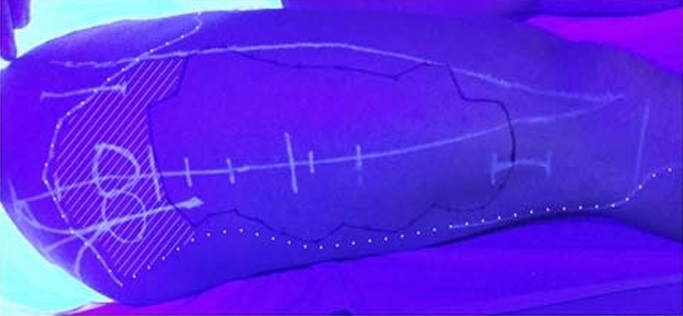
FIGURE 9. Photography of the lateral thigh while illuminated with
UV light on day 2 of the clinical trial. The shaded area represents
an example of a failure to anesthetize the proximal branches of
the LFC nerve. There is approximately 10 cm between the
proximal border of the area anesthetized by the SI-FIC block
(superimposed white dotted line) and the proximal border of the
area anesthetized by the LFC nerve block (solid black line). In this
image, the proximal margin of the SI-FIC block is atypical as it
extends beyond the average. In the majority of subjects, the
proximal border of the anesthetized area was distal to the greater
trochanter.
and in 11% to 18%, an LFC nerve branch reaches the proximal
thigh by crossing the iliac crest posterior to the ASIS.9,10 The re
sults of our dissection study support that the LFC nerve branches
crossing the ASIS can be anesthetized with an injection into the
FFFT. However, branches crossing the iliac crest posterior to the
ASIScannot bereachedbythisnerve blocktechnique. To the best
of our knowledge, a nerve block technique that anesthetizes these
branches has not yet been published other than presumably the
fascia iliaca compartment technique, anesthetizing the LFC nerve
in its intrapelvic trajectory prior to any branching. Consequently, a
failure rate of 10% to 20% for the LFC nerve block based on in
jection into the FFFT was expected a priori. In other words, we
could not expect more than 80% success rate of inclusion of the
most proximal branches of the LFC nervewith the FFFT technique.
The discrepancy between the expected and observed success rates
(80% and 68.4%, respectively) is due to either suboptimal proximal
spread of local anesthetic with the FFFT technique, suboptimal per
formance of the FFFT technique, too short observation time after
block performance, or measurement error.
Weallowed a measurement error of 3 cm per protocol in the
comparison of proximal margin of the anesthetized area between
the 2 techniques based on pilot data. However, our repeated esti
mates of the proximal margin of the anesthetized area with the
SI-FIC nerve block technique demonstrated that this error was
too narrow to allow the intended type 1 measurement error of
5%. If the correct fifth percentile of the duplicate measurements
(−7.5 cm) were used as the allowed deviation for our primary
end point, the success rate would be 79% (15/19; 95% CI, 54%
94%) instead of the 68%.
Some other limitations to the volunteer trial should be men
tioned: In thevolunteer trial, the distance from the needle insertion
point to the ASIS was not measured. If spread outside the FFFTis
obtained, which is positively correlated to the volume of injectate
according to the dissection study, then the level of needle insertion
presumably affects the proximal extent of this spread. However, a
significant difference in the mean distance between active and
placebo sides would be unlikely because of the randomized and
blinded design.
Moreover, the volunteer trial was conducted in a group of
subjects who differed concerning age, weight, and comorbidities
compared with the typical hip surgery patients. Even though individ
ual patterns of innervation are not believed to change through life and
should therefore not affect the primary end point, other end points
may show to be different in a group of patients, for example, perfor
mance time and quality of ultrasonographic visualization.
Finally, we used the SI-FIC block as a criterion standard of
anesthesia of all branches of the LFC nerve. The proximal border
of the anesthetized area after the LFC nerve block was compared
with this criterion standard. If the SI-FIC block does not reliably
anesthetize all LFC nerve branches, the success rate of the LFC
nerve block of anesthetizing the proximal LFC nerve branches
would be overestimated. Our validation of the technique is based
on a previous dissection study of the SI-FIC block technique. In
that study, coloring of the LFC nerve in its intrapelvic trajectory
was assessed to be reliable in all cadaver sides.23 This finding
wasfurther supported by a morerecentstudyof clinicalanesthesia
of the lateral thigh.32 Anesthesiawas found in all subjects in a case
series of patients receiving a suprainguinal fascia iliaca block. The
100% bilateral success rate of lateral anesthesia after the SI-FIC
block performed in the current study additionally adds to the val
idation of the SI-FIC block to reliably anesthetize the LFC nerve.
Blockade of the nerves innervating the skin area proximal to
the area innervated by the LFC nerve could serve as an alternative
methodofassessing the success rate of anesthetizing the proximal
LFC nerve branches in future research trials. Continuity of the area anesthetized by the 2 nerve blocks would allow estimation
of the frequency of anesthesia of the proximal LFC nerve
branches. In addition, anesthesia of the area proximal to the area
innervated by the LFC nerve is a clinically relevant issue in order
to anesthetize the skin intersected by most surgical incisions as
demonstrated by the present study.
The present study demonstrates that cadaveric injection of
10 mL of dye in the FFFTwith dynamic needle-tip tracking to
ward the ASIS under ultrasound guidance produces spread of
dye to the proximal branches of the LFC nerve.
Applying this novel LFC nerve block technique in the ran
domized volunteer trial demonstrated that this new nerve block
technique was safe and easy to perform with a success rate of
anesthetizing the LFC nerve of 95%. The area of clinical anesthe
siawas distal tothe proximal borderofthegreater trochanterin the
majority of the subjects. The surgical incisions extending proximal
to the greater trochanter were typically not completely covered by
the LFC nerve block. The most proximal LFC nerve branches were
anesthetized with a success rate of 68% when the SI-FIC block was
used as a reference of the entire LFC nerve territory of innervation.
Further research is needed to obtain complete cutaneous anesthesia
of the areas intersected by the different hip surgery approaches.
1. Coad NR. Post-operative analgesia following femoral-neck surgery—a
comparisonbetween 3 in1 femoralnerve blockand lateral cutaneous nerve
block. Eur J Anaesthesiol.1991;8:287–290.
2. Jones SF. Analgesia following femoral neck surgery. Lateral cutaneous
nerve block as an alternative to narcotics in the elderly. Anaesthesia.1985;
40:682–685.
3. Segado Jiménez MI, Bayón Gago M, Arias Delgado J, et al. Efficacy of
obturator and femoralcutaneous nerve blocksfor postoperative analgesia in
hip surgery. Rev Esp Anestesiol Reanim. 2009;56:590–597.
4. SegadoJiménezMI.Post-surgicalanalgesiainhipsurgery:acomparisonof
three techniques [in Spanish]. Rev Esp Anestesiol Reanim.2010;17:
259–267.
5. Vandebroek A, Vertommen M, Huyghe M, Van Houwe P. Ultrasound
guided femoral nerve block and lateral femoral cutaneous nerve block for
postoperative pain control after primary hip arthroplasty: a retrospective
study. Acta Anaesthesiol Belg.2014;65:39–44.
6. Ghabach MB, Elmawieh JM, Matta MS, Helou MR. Combined block of
the femoral and lateral femoral cutaneous nerves under ultrasound for
post-operative analgesia in patients undergoing hip surgery: a double blind
randomized trial. Middle East J Anaesthesiol. 2016;23:421–426.
7. Thybo KH, Mathiesen O, Dahl JB, Schmidt H, Hagi-Pedersen D. Lateral
femoral cutaneous nerve block after total hip arthroplasty: a randomised
trial. Acta Anaesthesiol Scand. 2016;60:1297–1305.
8. Grothaus MC, Holt M, Mekhail AO, Ebraheim NA, Yeasting RA. Lateral
femoral cutaneous nerve: an anatomic study. Clin Orthop Relat Res.2005:
164–168.
9. RudinD,ManestarM,Ullrich O,ErhardtJ, GrobK.The anatomicalcourse
of the lateral femoral cutaneous nerve with special attention to the anterior
approach to the hip joint. J Bone Joint Surg Am. 2016;98:561–567.
10. Kosiyatrakul A,NuansaleeN,LuenamS,KoonchornboonT,PrachapornS.
The anatomical variation of the lateral femoral cutaneous nerve in relation
to the anterior superior iliac spine and the iliac crest. Musculoskelet Surg.
2010;94:17–20.
11. Hara K, Sakura S, Shido A. Ultrasound-guided lateral femoral cutaneous
nerve block: comparison of two techniques. Anaesth Intensive Care.2011;
39:69–72.
©2018 American Society of Regional Anesthesia and Pain Medicine 365
Copyright © 2018 American Society of Regional Anesthesia and Pain Medicine. Unauthorized reproduction of this article is prohibited.
Nielsen et al
Regional Anesthesia and Pain Medicine • Volume 43, Number 4, May 2018
12. Hurdle MF, Weingarten TN, Crisostomo RA, Psimos C, Smith J.
Ultrasound-guided blockade of the lateral femoral cutaneous nerve:
technical description and review of 10 cases. Arch Phys Med Rehabil.
2007;88:1362–1364.
13. Ng I, Vaghadia H, Choi PT, Helmy N. Ultrasound imaging accurately
identifies the lateral femoral cutaneous nerve. Anesth Analg. 2008;107:
1070–1074.
14. Tagliafico A, Serafini G, Lacelli F, Perrone N, Valsania V, Martinoli C.
Ultrasound-guided treatment of meralgia paresthetica (lateral femoral
cutaneous neuropathy): technical description and results of treatment in
20 consecutive patients. JUltrasoundMed. 2011;30:1341–1346.
15. Bodner G, Bernathova M, Galiano K, Putz D, Martinoli C, Felfernig M.
Ultrasound of the lateral femoral cutaneous nerve: normal findings in a
cadaver and in volunteers. RegAnesthPainMed. 2009;34:265–268.
16. Corujo A, Franco CD, Williams JM. The sensory territory of the lateral
cutaneous nerve of the thigh as determined by anatomic dissections and
ultrasound-guided blocks. Reg Anesth Pain Med. 2012;37:561–564.
17. Davies A,Crossley A, Harper M,O’Loughlin E.Lateral cutaneous femoral
nerve blockade–limited skin incision coverage in hip arthroplasty. Anaesth
Intensive Care. 2014;42:625–630.
18. Hopkins PM, Ellis FR, Halsall PJ. Evaluation of local anaesthetic blockade
of the lateral femoral cutaneous nerve. Anaesthesia.1991;46:95–96.
19. Thiel W. Photographischer atlas der praktischen anatomie.Berlin,
Germany: Springer-Verlag; 1996.
20. Zhu J, Zhao Y, Liu F, Huang Y, Shao J, Hu B. Ultrasound of the lateral
femoral cutaneous nerve in asymptomatic adults. BMC Musculoskelet
Disord. 2012;13:227.
21. FondiMA,NavaS,PosteraroCM,Vigorita I,Alessandri F, Dauri P. Lateral
femoral cutaneous nerve (LFCN): invivo anatomical study and ultrasound
(US) imaging: 823 [poster]. RegAnesthPainMed. 2008;33:101.
366
22. Hanna A. The lateral femoral cutaneous nerve canal. JNeurosurg.2017;
126:972–978.
23. Hebbard P, Ivanusic J, Sha S. Ultrasound-guided supra-inguinal fascia
iliaca block: a cadaveric evaluation of a novel approach. Anaesthesia.2011;
66:300–305.
24. McHanwell S, Brenner E, Chirculescu A, et al. The legal and ethical
framework governing body donation in Europe—a review of current practice
and recommendations for good practice. Eur J Anat. 2008;12:1–24.
25. Riederer BM, Bolt S, Brenner E, et al. The legal and ethical framework
governingbodydonationinEurope—1st update on current practice.
Eur J Anat.2012;16:1–21.
26. Kessler J, Moriggl B, Grau T. Ultrasound-guided regional anesthesia:
learning with an optimized cadaver model. Surg Radiol Anat.2014;36:
383–392.
27. Platzer W, Reinhard P, Pousel S. Ein neues konservierungs-und
aufbewahrungssystem für anatomisches material. Acta Anat. 1978;102:
60–67.
28. Chechik O, Khashan M,Lador R,Salai M,AmarE.Surgical approachand
prosthesis fixation in hip arthroplasty world wide. Arch Orthop Trauma
Surg. 2013;133:1595–1600.
29. Palm H, Posner E, Ahler-Toftehøj HU, et al. High reliability of an algorithm for
choice of implants in hip fracture patients. Int Orthop. 2013;37:1121–1126.
30. Bohannon RW. Measuring knee extensor muscle strength. Am J Phys Med
Rehabil.2001;80:13–18.
31. CasatiA,Putzu M.Bupivacaine, levobupivacaine and ropivacaine: are they
clinically different? Best Pract Res Clin Anaesthesiol. 2005;19:247–268.
32. Bullock WM, Yalamuri SM, Gregory SH, Auyong DB, Grant SA.
Ultrasound-guided suprainguinal fascia iliaca technique provides benefit as
an analgesic adjunct for patients undergoing total hip arthroplasty.
JUltrasoundMed. 2017;36:433–438.
