Abstract: Postoperative pain after bariatric surgery can be significant and yet difficult to manage. These patients frequently have associated obstructive sleep apnea and are at risk of respiratory depression with opioid anal gesia. Abdominal wall blocks such as the subcostal transversus abdominis plane block are not of significant benefit, probably in part because they provide only somatic analgesia. The ultrasound-guided erector spinae plane (ESP) block is a recently described regional anesthetic technique for providing thoracic analgesia when performed at the level of the T5 transverse process. Local anesthetic injected into the fascial plane deep to the erector spinae muscle spreads in a craniocaudal fashion over several levels. Local anesthetic also penetrates anteriorly through the inter transverse connective tissue and enters the thoracic paravertebral space where it can potentially block not only the ventral and dorsal rami of spinal nerves but also the rami communicantes that transmit sympathetic fibers. Coupled with the fact that the erector spinae muscle and ESP extend down to the lumbar spine, this suggests that the ESP block could result in both visceral and somatic abdominal analgesia if the injection were performed at a lower thoracic level. We describe a series of 3 cases that illustrate the efficacy of bilateral ESP blocks performed at the level of the T7 transverse process for relieving visceral abdominal pain following bariatric surgery. Further investigation is recommended to establish the potential of the ESP block as an analgesic modality in abdominal surgery.
(Reg Anesth Pain Med 2017;42: 372–376)
The ultrasound-guided erector spinae plane (ESP) block is a recently described technique for providing thoracic analgesia.1 Cadaveric investigation indicates that injection of 20-mL solution into the fascial plane deep to the erector spinae muscle at the level of the T5 transverse process can result in injectate spread between the C7 and T8vertebral levels. Given that the erector spinae muscle extends inferiorly to the lumbar spine, injection into the ESP at a lower vertebral level (eg, T7 or T8) should result in spread to the lower thoraco abdominal nerves that innervate the abdomen.In addition, because the mechanism of action of the ESP block in volves penetration of local anesthetic into the thoracic paravertebral space,1 it anesthetizes not only the ventral rami of spinal nerves but also the rami communicantes that contain sympathetic nerve fibers. The ESP block thus has the potential to provide both somatic and visceral sensory blockade, which would make it an ideal regional anesthetic technique for abdominal surgery. In this report, we provide preliminary clinical evidence to support this hypothesis with the description of successful use of bilateral ESP blocks at the level of the T7 transverse process to provide postoperative analgesia following laparoscopic bariatric surgery.
Written informed consent was obtained from all patients for inclusion in this report. Institutional research ethics board approval for case reports was not required by Toronto Western Hospital and the University Health Network.
Patient 1
A 35-year-old woman presented for elective laparoscopic roux-en-Y gastric bypass surgery. She was 163 cm tall, weighed 155 kg, and had a body massindex (BMI) of58.3 kg/m2.Notable comorbidities included mild asthma, recently diagnosed obstructive sleep apnea for which she was on continuous positive airway pressure (CPAP) therapy, a benign atrioventricular re-entrant tachycardia treated with sotalol 80 mg twice daily, mild mitral and tricuspid regurgitation, and mild pulmonary hypertension. General anesthesia was induced with propofol 200 mg, fentanyl 150 μg, remifentanil 100 μg, and rocuronium 60 mg and maintained with sevoflurane in an air-oxygen mixture and remifentanil infusion at 0.08 μg/kg per minute. Mild bronchospasm occurred following intubation, which resolved with sevoflurane. She received 2 further boluses of fentanyl 50 μg intravenously (IV) for intraoperative analgesia. The laparoscopic port sites were infiltrated with 20 mL of 0.25% bupivacaine with 5 μg/mL epinephrine. The surgery itself was uneventful, and the patient was extubated awake but drowsy. In the postanesthetic care unit (PACU), she remained drowsy, had several minor episodes of air way obstruction, and was consequently placed onnasal CPAP. De spite her drowsiness, the patient complained of severe deep epigastric pain for which she received 2 doses of fentanyl 25 μg IV that made her more somnolent without providing significant pain relief. In an attempt to provide rescue analgesia with out exacerbating her respiratory depression, bilateral ultrasound-guided posterior quadratus lumborum blocks2,3 with 20 mL of 0.5% ropivacaine per side were performed in the supine position 120 minutes after her arrival in PACU.
However, onassessment 30 minuteslater, the patient still had deep epigastric pain of 10/10 severity on a numerical rating scale (NRS). Hydromorphone 0.4 mg IV was administered, but this again significantly increased her somnolence and lowered her ox
ygen saturation without relieving her pain. At this point, we de cided to perform bilateral ESP blocks in a final attempt to provide adequate analgesia with out further respiratory depression.
The patient was turned into a left lateral position while maintaining a 45-degree head-up angle to facilitate diaphragmatic excursion and respiration. The level of the T7 rib and transverse process was located using ultrasound and a counting-down approach from the
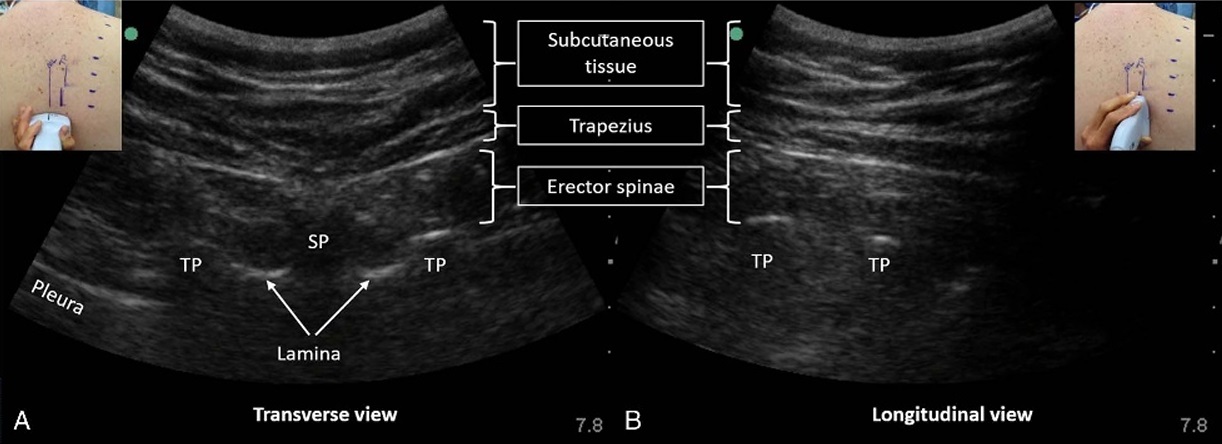
FIGURE 1. Identifying the tip of the transverse process in an obese subject. The level of the targeted transverse process (T7 in this case) is determined by surface landmarks or using ultrasound and counting up from the 12th rib or down from the first rib. The ultrasound transducer is first placed in (A) a transverse orientation to visualize the spinous process (SP) and the tips of the transverse processes (TP). The positions of these are marked on the skin. The transducer is then rotated into (B) a longitudinal orientation in the parasagittal plane of the TPs. This is the view used to perform the ESP block. The erector spinae muscle and trapezius are visible superficial to the TPs. The rhomboid majormuscletapers off and ends at or abovethelevel of the T6 TP and is the reforenotvisible. Note that the pleura isalso not usually visible in this view because its surface is curving away from the transducer and is almost parallel to the ultrasound beam.
first rib; this was marked on the skin. The skin was disinfected with 2% chlorhexidine in 70% alcohol and a 2- to 5-MHz curved-array ultrasound transducer (SonoSite Edge, Bothell, Washington) was placed in a transverse orientation to identify the right lateral tip of the T7 transverse process (Fig. 1). The transducer was then rotated 90 degrees into a longitudinal parasagittal orientation over the transverse processes. The trapezius and erector spinae muscles were identified superficial to the acoustic shadow of the transverse processes; the absence of rhomboid major muscle was used as further confirmation that the transverse processes below T6 were being imaged (Fig. 1). A 22-gauge, 80-mm block needle (Pajunk, Geisingen, Germany) was inserted in-plane to the ultrasound beam in a superior-to-inferior direction to contact the T7 transverse process. Hydrolocation with 0.5 to 1 mL of 2% lidocaine was used to confirm needle tip position
in the ESP deep to erector spinae muscle; this was indicated by linear fluid spread that lifted the erector spinae muscle off the underlying transverse processes and intercostal muscles (Fig. 2). Twenty milliliters of a solution comprising 10 mL normal saline, 5 mL2%lidocaine, and 5 mL 1% ropivacaine was injected. This solution was chosen to balance the desire for a rapid onset of block and reasonable duration of action against the risk of local anesthetic systemic toxicity posed by the earlier administration
of 200 mg ropivacaine in the quadratus lumborum blocks. The
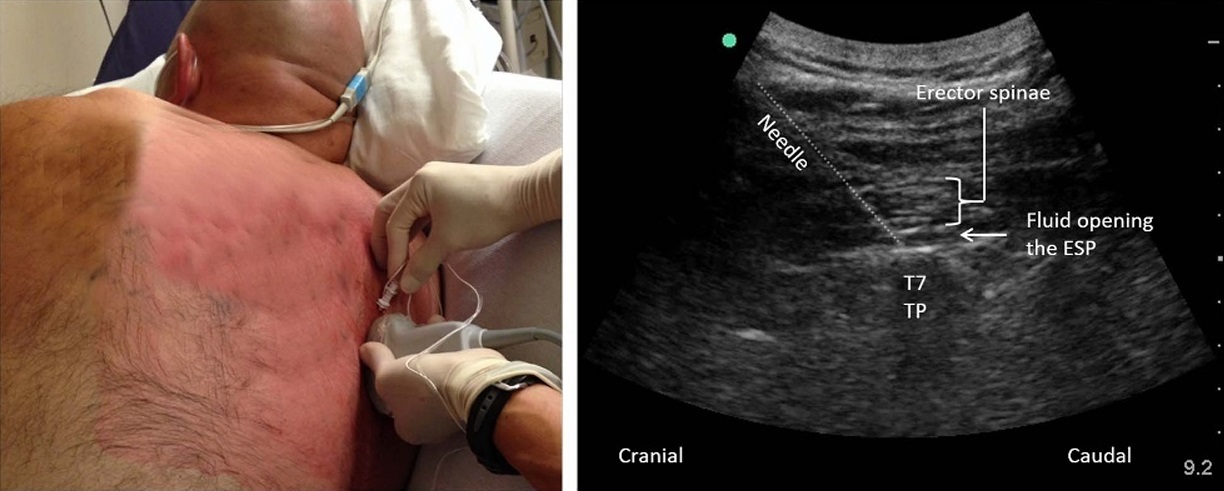
FIGURE 2. Performance of the ESP block in the second patient of the case series. Bilateral blocks were performed with the patient in a right lateral position with a head-up angle to facilitate diaphragmatic excursion and respiration. The needle is inserted in a cranial-to-caudal direction in-plane with the ultrasound beam to contact the T7 transverse process. Needle tip placement in the correct plane deep to erector spinae muscle is confirmed by visualization of a linear pattern of fluid spread that lifts the erector spinae muscle off the transverse process.
procedure was repeated in an identical manner on the left side without having to turn the patient onto her other side. Within 5 minutes of completion of the ESP blocks, the patient reported that her NRS pain score was 1/10. She was monitored in PACU
for a further hour to exclude local anesthetic systemic toxicity, during which time she remained comfortable and easily rousable with oxygen saturations of 95% or more on nasal CPAP. Her first request for analgesia occurred 2 hours after the ESP blocks in the form of IV hydromorphone; she received further doses as requested approximately every 4 to 5 hours. Her analgesic management was complicated by severe nausea, which led her to refuse oral acetaminophen, but her abdominal pain nevertheless remained well controlled at an NRS pain score of 3 to 4/10 during her stay. Her opioid requirements were 5.5 mg IV hydromorphone (27.5 mg IV morphine equivalents2) in 24 hours for the first 48 hours following the ESP blocks. Shewas discharged from hospital on the third postoperative day.
Patient 2
A43-year-old man, 179 cm in height and weighing 171 kg (BMI 53.3 kg/m2) underwent elective laparoscopic roux-en-Y gastric surgery. His medical issues included obstructive sleep apneamanaged with out CPAP therapy,chronic shoulder painrequiring pregabalin 100 mg and amitriptyline 50 mg at bedtime, and hypertension controlled with perindopril. He received a routine general anesthetic that was maintained with a desflurane-air mixture. Intraoperative analgesia consisted of fentanyl 150 μgI Vat
induction followed by an IV remifentanil infusion at 0.05 μg/kg per minute, ketorolac 30 mg IV, and 4 further boluses of fentanyl 50 μg IV. The laparoscopic port sites were infiltrated with 20 mL of 0.25% bupivacaine with 5 μg/mL epinephrine. Anesthetic emergence and extubation were uncomplicated, and the patient was transferred to the PACU, where he reported moderate incisional and back pain (5 to 7/10 on an NRS) and received fentanyl 100 μg IV and hydromorphone 0.5 μgI Vwith good effect. Approximately 90 minutes into his PACU stay and shortly before hewas due to be discharged, the patient had sudden onset of deep supraumbilical abdominal pain, which he described as “gas-like” in nature and 8/10 in severity. He was given IV hydromorphone 0.5 mg with little effect. After a further 30 minutes of observation, during which time the pain continued unabated, we proposed performing bilateral ESP blocks for analgesia based on our experience with the previously described case, to which
the patient agreed. He was turned into a right lateral position, and ESP blocks were performed as described previously at the level of the T7 transverse process (Fig. 2). Twenty milliliters of 0.5% ropivacaine was injected on each side. The patient reported
a rapid and noticeable improvement in his NRS pain score to 6/10 at 5 minutes after block completion. When he was assessed again 40 minutes later, the deep abdominal pain had decreased to 2/10 in intensity without further systemic analgesia, and he was
discharged from PACU to the general ward. His first request for additional analgesia occurred 7 hours after the ESP blocks, be cause of recurrence of the deep abdominal pain, for which he was given IV morphine. On review, the next morning, the patient
reported undisturbed sleep with the severity of abdominal pain no higher than 3/10. Hewas unable to discern a specific time that the block might have worn off. His pain subsequently remained well controlled on regular acetaminophen and oral oxycodone as needed in addition to his usual dose of pregabalin and amitriptyline for his other chronic painissues. His total opioidrequirements (expressed as IV morphine equivalents) in the first and second 24-hour periods following the ESP blocks were 12 and 10 mg, respectively. He was discharged on the second postoperative day without complication.
Patient 3
A 65-year-old man was scheduled for elective laparoscopic sleeve gastrectomy. He was 172 cm tall and weighed 166 kg, with a BMI of 56.1 kg/m2. His significant medical issues included severe obstructive sleep apnea requiring nocturnal biphasic positive
airway pressure therapy, significant orthopnea, chronic obstructive airways disease with a productive cough, fibromyalgia man aged with pregabalin 300 mg twice daily, insulin-dependent type 2 diabetes mellitus, and hypertension. In view of his respiratory conditions and body habitus, it was planned that he would be ad mitted postoperatively to the intensive care unit for close monitoring. In addition, it was agreed with the patient that wewould insert bilateral ESP catheters preoperatively to reduce the risk of postop
erative pain and opioid-induced deterioration in respiratory function. Thoracic epidural analgesia was not considered because of anticipated technical difficulty and the need for postoperative venous thromboprophylaxis. The ESP blocks were carried out in a
dedicated block room area, with the patient in a sitting position.
The blocks were performed at the level of the T7 transverse process using the technique described in the first case but using acat heter-over-needle peripheral nerve catheter set (Pajunk E-Cath, Geisingen, Germany). Twenty milliliters of 0.5% ropivacaine was injected on each side. The patient subsequently received a routine general anesthetic with a desflurane-air mixture. Intraoperative analgesia consisted of fentanyl 150 μg IVat induction and an IV remifentanil infusion at 0.05 μg/kg per minute. No further doses of fentanyl were administered. The laparoscopic port sites were
infiltrated with 20 mL of 0.25% bupivacaine with 5 μg/mL epi nephrine at the end of surgery. Emergence from anesthesia and extubation were uncomplicated, and the patient was transferred to the intensive care unit for monitoring as planned. He had no epigastric discomfort, and there was sensory loss to pinprick over the T7 to T11 dermatomes. Logistic and nursing constraints prevented the use of continuous local anesthetic infusion through the catheters, and a regimen of intermittent boluses as needed was initiated instead. At follow-up assessment 8 hours postoperatively, the patient had minimal epigastric pain, but instead complained of pain in the lower abdominal and suprapubic area, which he rated as 8/10 on an NRS. A bolus of 20 mL of 0.5% ropivacaine was administered through each catheter, which reduced the pain
to 5/10. The patient remained in hospital for the next 3 days, dur ing which time he was followed by the acute pain service team. The main analgesic issue continued to be “deep” pain in the in fraumbilical and suprapubic area which, although unclear in
its etiology, was deemed to be of little cause for surgical concern. There was no significant upper abdominal pain. He received an additional bolus of 20 mL0.5% ropivacaine through eachcatheter on the morning of the first and second postoperative days, which relieved his lower abdominal pain from 8 to 9/10 to 3/10 for several hours. Total opioid requirements (expressed as IV morphine equivalents) were 17.5 mg in the first 24 hours postoperatively and 22.5 mg in the subsequent 24 hours. The catheters were re moved after the top-up on the second post operativ dayas the sites
were slightly tender. The patient received additional oral hydro morphone totaling 28 mg of IV morphine equivalents before being discharged from hospital on the third postoperative day. There were no respiratory complications during his stay.
Bariatric surgery is increasingly common in North America, and despite the laparoscopic approach, up to 42% of patients ex perience severe pain (NRS>7/10)at least once andrequire a mean of 73 mg of IV morphine in the first 48 hours after surgery.3 So
matic pain from the laparoscopic port sites is readily treated with local anesthetic infiltration; however, visceral epigastric pain can be more difficult to manage. The use of opioids is limited by concerns regarding patient somnolence at emergence, as well as post operative respiratory depression,4 particularly given the high prevalence of obstructive sleep apnea in this population. Other reasons to minimize opioid use include avoiding the adverse effects of paralytic ileus and nausea and vomiting. Nonsteroidal anti inflammatory drugs are often avoided due toreported associations with gastrointestinal anastomotic leaks5 and the development of marginal ulcers,6 although the risk from a single dose is unclear.
Regional anesthetic techniques are a logical analgesic modality but have not been widely studied inthis setting. Continuous in traperitoneal and subfascial/subcutaneous infusion of bupivacaine at wound sites has been investigated in several studies and found to have only modest analgesic efficacy.7–9 Subcostal transversus abdominis plane (TAP) blocks have not been shown to offer any additional benefit over systemic multimodal analgesia in this patient population,10 possibly because of the fact that TAP blocks provide only somatic analgesia. The quadratus lumborum block is an alternative abdominal wall block that purports to act by local anesthetic spread to the thoracic paravertebral area and to provide coverage up to the T7–9 dermatomes.11 Clinical studies supportits analgesic efficacy in lower-segment cesarean section,12,13 but
at present, there is a paucity of clinical data to indicate if analgesia of the upper abdomen can be consistently achieved. Furthermore, imaging the area of the quadratus lumborum muscle can be very difficult in the obese population. Both these factors may have con tributed to the lack of effect observed from quadratus lumborum
blocks in our first patient.
The ESP block, on the other hand, provided dramatic relief of upper abdominal visceral pain in the first 2 patients in this series. Our third patient received preoperative and postoperative ESP blocks and had minimal upper abdominal pain during the course of his perioperative care. Furthermore, the ESP blocks were effective in relieving the unusual lower abdominal pain that he ex perienced; the etiology of this pain was unclear but from its description is likely to have been visceral in origin. We have previously demonstrated in a cadaver model that local anesthetic injected deep to the erector spinae muscle penetrates anteriorly through the connective tissues and ligaments spanning the adjacent transverse processes and enters the thoracic paravertebral space.1 The ESP block canthusanesthetize not only theventral and dorsalramiofthe spinal nerve roots, but also the rami communicantes that transmit au
tonomic fibres to and from the sympathetic ganglia (Fig. 3), resulting in both somatic and visceral analgesia. This contrasts with other more peripheral abdominal wall blocks such as the TAP block, which act only on somatic branches of the spinal nerves and may also miss the posterior and lateral cutaneous branches. The ESP block was originally described for thoracic analgesia when performed at the T5 transverse process1; however, these

FIGURE 3. Anatomy of the ESP block. Local anesthetic (in blue) injected anterior (deep) to the erector spinae muscle (ESM) spreads in a craniocaudal direction along this tissue plane. It also spreads anteriorly through the connective tissue spanning the transverse processes (TP) and enters the thoracic paravertebral space to anesthetize not only the ventral ramus and dorsal ramus of the spinal nerve, but also the white and gray rami communicantes that carry the preganglionic and postganglionic sympathetic fibers to and from the sympathetic ganglia. The ESP block thus has the potential to provide both somatic and visceral analgesia of the trunk. The proximal site of action blocks afferents from posterior, lateral, and anterior branches of the ventral ramus, resulting in extensive somatic analgesia. The TAP block, by contrast, acts more peripherally and provides only somatic analgesia to the anterior abdomen. RMM indicates rhomboid major muscle; TM, trapezius muscle; TP, transverse process. Adapted and reproduced with permission from Maria Fernanda Rojas Gomez.
cases, together with a recently published report of its application in laparoscopic ventral hernia repair,14 indicate that performing the ESP block at a lower vertebral level such as T7 can also provide effective and extensive abdominal analgesia. This is explained
by the fact that the erector spinae muscle is a paraspinous column of muscle that extends from its origins from the sacrum and lum bar spinous processes to its insertions in the thoracic and cervical spine. It is encased along its length in a fascial retinaculum (the thoracolumbar fascia),15 which provides a conduit for cranio caudal spread of local anesthetic to multiple thoracic and even lumbar vertebral levels.14 This is consistent with the sensory block from T 7 to T 11 observedinour thirdpatient, aswellas the clinical
relief of his lower abdominal pain. Whileasimilaranalgesic effect might be obtainedwitha thoracic epidural or bilateral paravertebral blocks, the ESP block of fers significant advantages in terms of ease of performance and safety. The transverse processes of the thoracic vertebrae and the erector spinae muscles are easily identified even in obese patients, using a low-frequency curved-array transducer; there is a clear
endpoint for needle insertion and injection; and the risk of pneu mothorax is minimal because the needle does not need to advance past the posterior surface of the transverse process (Fig. 3). Fur thermore, as illustrated here, it can be readily performed in the lateral position, making it a feasible postoperative “rescue” block. As
with all single-shot regional anesthesia techniques, the fixed duration of analgesia is a limitation. The short duration of analgesia observed in our first patient can be attributed to the dilute lidocaine-ropivacaine mixture that was chosen to minimize the risk of local anesthetic systemic toxicity from cumulative dosing. The duration of analgesia observed with a 20-mL bolus of 0.5% ropivacaine in our second and third patients, on the other hand, is consistent with that reported for other abdominal wall blocks.16 Nevertheless, the fascial compartment of the ESP lends itself well to catheter insertion, which can be used toprolong the effect of the block when required, as illustrated by our third case.
The optimal dosing regimen for ESP catheters, be it continuous infusion or intermittent boluses, remains to be determined, however. Finally, the relatively superficial location of the ESP block, distant from any major blood vessels and nervous structures, also mini
mizes concerns regarding anticoagulation and the development of a clinically significant hematoma. In summary, these 3 cases illustrate that the ESP block may hold potential as a relatively simple regional anesthesia technique for providing both visceral and somatic analgesia after abdominal surgery. Further clinical investigation, including prospective ran domized controlled trials, should be undertaken to clearly establish its efficacy in this setting.
1. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae
plane block: a novel analgesic technique in thoracic neuropathic pain.
Reg Anesth Pain Med. 2016;41:621–627.
376
2. Patanwala AE, Duby J, Waters D, Erstad BL. Opioid conversions in acute
care. Ann Pharmacother. 2007;41:255–266.
3. Weingarten TN, Sprung J, Flores A, Baena AMO, Schroeder DR,
Warner DO. Opioid requirements after laparoscopic bariatric surgery.
Obes Surg. 2011;21:1407–1412.
4. Mulier JP. Perioperative opioids aggravate obstructive breathing in sleep
apnea syndrome: mechanisms and alternative anesthesia strategies. Curr
Opin Anaesthesiol. 2016;29:129–133.
5. Subendran J, Siddiqui N, Victor JC, McLeod RS, Govindarajan A.
NSAID use and anastomotic leaks following elective colorectal
surgery: a matched case-control study. J Gastrointest Surg.2014;18:
1391–1397.
6. SverdénE,MattssonF,SondénA,etal.Riskfactorsfor marginalulcerafter
gastric bypass surgery for obesity: a population-based cohort study.
Ann Surg. 2016;263:733–737.
7. Iyer CP, Robertson BD, Lenkovsky F, Huerta S, Livingston E, Thurmon JJ.
Gastric bypass and On-Qpump:effectiveness ofSoakerCathetersystemon
recovery of bariatric surgery patients. Surg Obes Relat Dis.2010;6:
181–184.
8. Symons JL, Kemmeter PR, Davis AT, et al. A double-blinded,
prospective randomized controlled trial of intraperitoneal bupivacaine in
laparoscopic roux-en-Y gastric bypass. JAmCollSurg. 2007;204:
392–398.
9. Moncada R, Martinaitis L, Landecho M, et al. Does preincisional
infiltration with bupivacaine reduce postoperative pain in laparoscopic
bariatric surgery? Obes Surg. 2016;26:282–288.
10. Albrecht E, Kirkham KR, Endersby RVW, et al. Ultrasound-guided
transversus abdominis plane (TAP) block for laparoscopic gastric-bypass
surgery: a prospective randomized controlled double-blinded trial.
Obes Surg. 2013;23:1309–1314.
11. Murouchi T, Iwasaki S, Yamakage M. Quadratus lumborum block:
analgesic effects and chronological ropivacaine concentrations
after laparoscopic surgery. Reg Anesth Pain Med. 2016;41:
146–150.
12. BlancoR,Ansari T, Girgis E.Quadratus lumborumblock for postoperative
pain after caesarean section: a randomised controlled trial. Eur J
Anaesthesiol. 2015;32:812–818.
13. Blanco R, Ansari T, Riad W, Shetty N. Quadratus lumborum block versus
transversus abdominis plane block for postoperative pain after cesarean
delivery: a randomized controlled trial. Reg Anesth Pain Med.2016;41:
757–762.
14. Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of
pre-operative bilateral erector spinae plane (ESP) blocks in patients having
ventral hernia repair. Anaesthesia. 2017;72:452–460.
15. Willard FH, Vleeming A, Schuenke MD, Daneels L, Schleip R. The
thoracolumbar fascia: anatomy, function and clinical considerations.
JAnat. 2012;221:507–536.
16. Chin KJ, McDonnell JG, Carvalho B, Sharkey A, Pawa A, Gadsden J.
Essentials of our current understanding: abdominalwall blocks. Reg Anesth
Pain Med. 2017;42:113–183.
