Background and Objectives: Fascial plane blocks are rapidly emerging to provide safe, feasible alternatives to epidural analgesia for thoracic and abdominal pain. We define a new option for chest wall and upper abdominal analgesia, termed the rhomboid intercostal and subserratus plane (RISS) block. The RISS tissue plane extends deep to the erector spinae muscle medially and deep to the serratus anterior muscle laterally. We describe a 2-part proof-of-concept study tovalidate the RISS block, including a cadaveric study to evaluate injectate spread and a retrospective case series to assess dermatomal coverage and analgesic efficacy.
Methods: For the cadaveric portion of the study, bilateral ultrasound guided RISS blocks were performed on 6 fresh cadavers with 30 mL of 0.5% methylcellulose with india ink. For the retrospective case series, we present 15 patients who underwent RISS block or RISS catheter insertion for heterogeneous indications including abdominal surgery, rib fractures, chest tube–associated pain, or postoperative incisional chest wall pain.
Results: In the cadaveric specimens, we identified staining of the lateral branches of the intercostal nerves from T3 to T9 reaching the posterior primary rami deep to the erector spinae muscle medially. In the clinical case series, dermatomal coverage was observed in the anterior hemithorax with visual analog pain scores less than5 in patients who underwent bothsingle shot and continuous catheter infusions.
Conclusions: Our preliminary cadaveric and clinical data suggest that RISS block anesthetizes the lateral cutaneous branches of the thoracic in tercostal nerves and can be used in multiple clinical settings for chest wall and upper abdominal analgesia.
(Reg Anesth Pain Med 2018;43: 745–751)
Interfacial plane blocks of the chest wall remain in early stages of development, but initial results show promise in offering alter natives to neuroaxial blocks.1,2
Chest wall blocks represent an umbrella term wherein a number of successful approaches have been described. The serratus anterior plane block3,4 has been used for analgesia after breast surgery,5–8
postmastectomy pain,9,10 thoracoscopic surgery,11,12 pain associated with rib fractures, shoulder surgery,13–16 open thoracotomy,17 and postthoracotomy pain.18–20 The erector spinae plane block21
has been used for acute postsurgical pain, posttraumatic pain 21,22 and chronic neuropathic pain conditions.21
We previously reported the extent of contrast spread in cadavers following the rhomboid intercostal block at one injection point in the triangle of auscultation.23 We observed injectate spread between the intercostal muscles and deep to the serratus anterior muscle, as well as staining of the lateral cutaneous branches of intercostal nerves T3toT8.Oneofthecharacteristics offascial systems is the continuity across different anatomical areas. For example, the tissue plane deep to the erector spinae muscle in the upper thoracic area is continuous with the tissue plane deep to the rhomboid major muscle, as well as the tissue plane deep to the serratus anterior muscle.24
In this study, we sought to investigate the injectate spread as a proof of concept for the continuity of posterior chest wall fascial systems in a cadaveric model and evaluate the clinical response to injection with a retrospective case series of rhomboid intercostal and subserratus plane (RISS) block. We hypo the sized that the rhomboid intercostal block can be extended caudally by positioning the needle tip deep to the serratus muscle to block the lateral cutaneous branches of intercostal nerves to T11 and still extend medially deep to the erector spinae muscle to block the dorsal rami. In addition, we believe that the location of the injection (upper thoracic to low thoracic) relative to the lateral cutaneous branches of the intercostal nerves may influence both injectate spread and clinical efficacy.
Cadaveric Study
Six unembalmed (fresh) adult cadavers representing a range of body habitus and both sexes were chosen. Cadavers with known thoracic deformities or previous spine surgery were excluded from the study. All cadavers were maintained at room temperature for 12 hours before injection. Bilateral ultrasound-guided RISS blocks were performed on each cadaver (n = 12 injections) by one investigator (H.E.).
Description of the Technique
The cadaveric specimens were placed in the prone position, with both arms abducted and internally rotated to move the inferior angle of the scapula laterally. A linear ultrasound transducer (6–12 MHz Mindray M7 diagnostic ultrasound system; Mindray DSUSAInc, Mahwah, New Jersey) was placed in the sagittal plane medial to the medial border of the scapula with the orientation
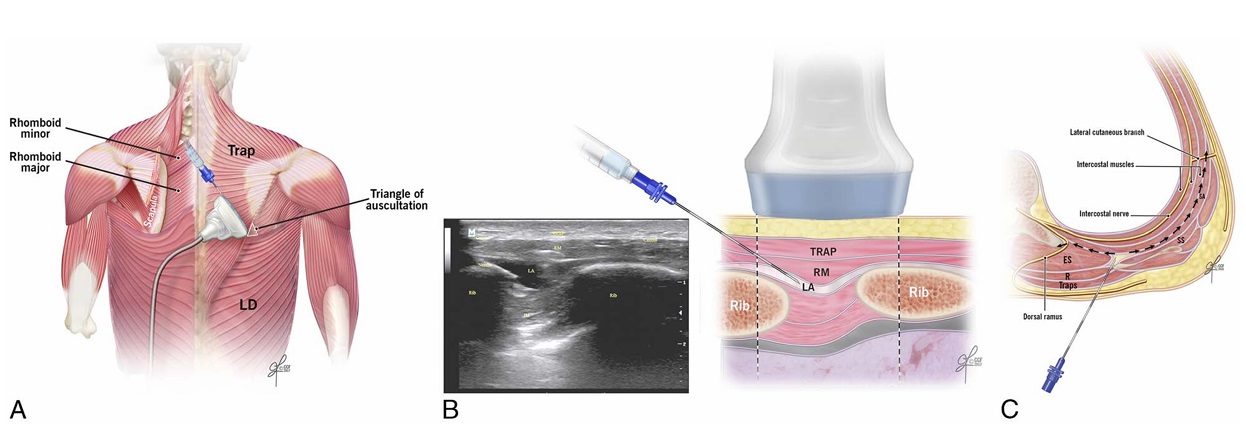
FIGURE1. A,Ultrasound transducer positioning for performance of the rhomboidintercostalinjection at theT5-6 level.LD indicateslatissimus dorsi muscle; Trap, trapezius muscle. B, Schematic illustration demonstrating surrounding structures and needle position for rhomboid intercostal injection at the T5-6 level (right) and corresponding ultrasound image (left). IM indicates intercostal muscles; LA, local anesthetic; RM,rhomboid majormuscle; Trap, trapezius muscle. C, Schematic illustration of an axial section at the level of T5-6 demonstrating needle position and injectate spread during rhomboid intercostal injection. ES indicates erector spinae muscle; R, rhomboid muscle; SA, serratus anterior muscle; SS, subscapularis muscle.
marker directed cranially. The transducer was then rotated so the cranial end was directed slightly medially and the caudal end laterally to produce an oblique sagittal view (paramedian sagittal oblique) approximately 1 to 2 cm medial to the medial
scapular border (Fig. 1A).
The following structures were identified from superficial to deep: trapezius muscle, rhomboid major muscle, intercostal muscles between ribs, pleura, and lung. The tissue plane between the rhomboid major and intercostal muscles was identified. A 17-gauge Tuohy needle was advanced in plane from a superomedial-to-inferolateral
direction, through the trapezius and rhomboid major muscles. Ten milliliters of 0.5% methylcellulose with india ink was injected in the fascial plane between the rhomboid major muscle and the intercostal muscles. The skin entry point for the first injection was at the T5–T6 level just medial to the scapula (Fig. 1, B and C; Video 1, Supplemental Digital Content 1, http://links.lww.com/AAP/A267). Two landmarks verified identification of the T5–T6 level: (1) counting down from the C7 spinous process and (2) identifying the medial part of the spine of the scapula at the T3 level. Next, to identify the subserratus plane, the transducer was moved caudally and laterally, distal to the inferior angle of the scapula behind the posterior axillary line (Fig. 2, A–C). Tissue layers were identified from superficial to deep: latissimus dorsi, serratus anterior, intercostal muscles between ribs, pleura, and lung (Muscle Layers, Supplemental Digital Content 2, http://links. lww.com/AAP/A268). The needle was inserted at the same skin entry site as was used for the rhomboid intercostal injection but directed caudally and laterally beyond the inferior angle of the scapula. If the needle tip did not reach beyond the inferior edge of the scapula (eg, obese and tall habitus), a new skin entry point
medial to the lower angle of the scapula and posterior axillary line was used. Twenty milliliters of 0.5% methylcellulose with india ink was injected in the tissue plane between the serratus anterior and external intercostal muscle, hydrodissecting the tissue plane between the serratus anterior muscle and the attachments of the serratus to the rib (Video, Supplemental Digital Content 3, http://links.lww. com/AAP/A269). The same procedure was repeated on the contralateral side.
Cadaveric Anatomic Dissection
The chest walls were dissected in layers from superficial (posterior) to deep (anterior) by an anatomist (R.L.D.) 2 hours fol lowing the injection. The detailed cadaveric dissection is available
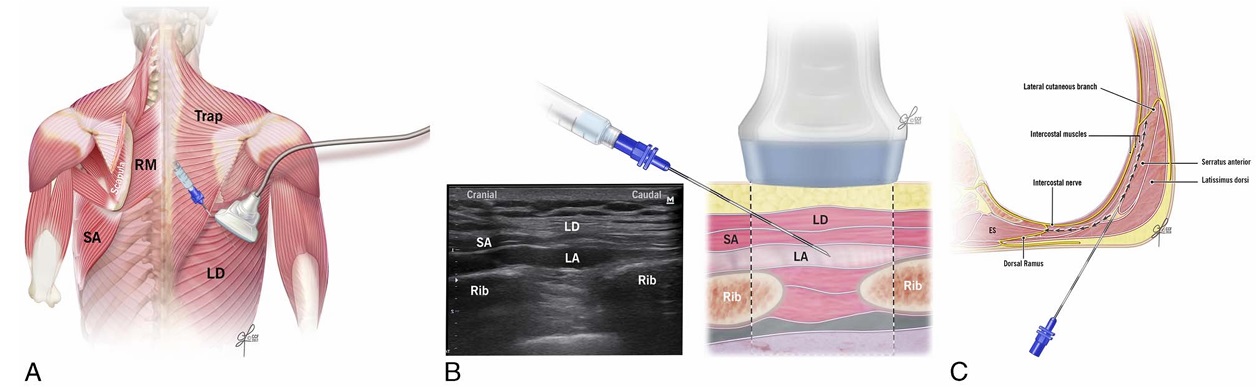
FIGURE 2. A, Ultrasound transducer positioning for subserratus plane injection at the T7-8 level. LD indicates latissimus dorsi muscle; RM, rhomboid major muscle; SA, serratus anterior muscle; Trap, trapezius muscle. B, Schematic illustration demonstrating surrounding structures and needle position for subserratus injection at the T7-8 level (right) and corresponding ultrasound image (left). LA indicates local anesthetic; LD, latissimus dorsi muscle; SA, serratus anterior muscle. C, Schematic illustration and sagittal ultrasound image during the performance of the subserratus block at T7-8 demonstrating the surrounding muscle layers and local anesthetic spread. ES indicates erector spinae muscle.
as supplemental material (Cadaveric Clarification, Supplemental Digital Content 4, http://links.lww.com/AAP/A270, and Supplemental Digital Content 5, http://links.lww.com/AAP/A271). We dissected the trapezius from its attachment on the spinous processes to expose the rhomboid major and minor muscles. Next, the latissimus dorsi muscle was reflected laterally. The erector spinae muscles were then
reflected cranially to identify the thoracic transverse processes and the origins of the dorsal rami.
The lateral cutaneous branches of the intercostal nerves were identified at the midaxillary line in the tissue plane deep to the serratus anterior muscle. Subsequently, ribs T2 to T12 were re moved at the articular surface of the transverse processes to visu
alize any staining in the intercostal spaces.
The axillary space was identified between the pectoralis minor muscleanteriorly and subscapularis muscle posteriorly. Below the eighth thoracic level, the upper part of the external oblique muscle was identified and removed to identify the tissue plane lo
cated superficial to the external intercostal muscles and traversing around the anterior axially line. All key steps of the dissection were photographed.
Clinical Case Series
The Cleveland Clinic Institutional Review Board approved a retrospective review of cases performed between April 2016 and August 2017.Written informed consent for the nerve blockproce dures were obtain fromall patients. Patients were positioned in the lateral decubitus position with the painful/operative side up and were monitored with standard American Society of Anesthesiolo gists monitoring. A rhomboid intercostal injection was performed using a 17-gaugeTuohyneedleutilizing the same techniqueasde scribed above in the cadaveric study. Five to 10 mL of local anes thetic (0.5% ropivacaine or bupivacaine) was administered to patients receiving a single-shot block or 5 to 10 mL of 0.2% ropivacaine in patients receiving a catheter. The rhomboid inter costal component of the block was performed as a single-shot injection at the level of T3 to T6, depending on desired dermatomal coverage, whereas the subserratus plane component was per formed next, as either a single-shot injection or continuous cathe
ter technique. The subserratus plane injection was performed in a similar fashion as described in the cadaveric model, with the needle target at the level of T4 to T10 (again depending on desired dermatomal coverage). When the subserratus plane was reached
(at the lower angle of the scapula), 15 to 20 mL of local anesthetic was administered (0.5% ropivacaine or bupivacaine for single shot blocks or 0.2% ropivacaine for patients receiving catheters). The target landmark was the plane located superficial to the inter costal muscles. The injectate was visualized to spread both crani ally and caudally throughout the subserratus tissue plane, noting the injectate volume pushing the serratus anterior muscle away from the external intercost al muscle (External Photos, Supple mental Digital Content 6, http://links.lww.com/AAP/A272).
In subjects undergoing a continuous infusion, a 19-gauge, 40-cm catheter (Arrow by Teleflex; Teleflex, Morrisville, North Carolina) was then introduced into the subserratus plane and advanced 3 to 5 cm beyond the needle tip. The catheter tip position was confirmed with injection of 5 mL of 0.2% ropivacaine under direct ultrasound visualization (Video, Supplemental Digital Content 7, http://links.lww.com/AAP/A273). Catheters were secured with sterile adhesive dressing.
Additional outcome data were obtained from the electronic medical record including the extent of sensory dermatomal coverage as determined by a loss of cold sensation to ice,duration of an algesia, and pain scores using visual analog score before and after the block.
RESULTS OF THE CADAVERIC STUDY
Six cadavers were included in the study (3 male, 3 female) with body mass indices between 28 and 44 kg/m2. We identified the tissue plane between the rhomboid major and minor muscles, serratus anterior muscle, external intercostal muscle, and external
oblique muscles from T2 to T12. In addition, we identified the lateral branches of the intercostal nerves from T2 to T12 and dorsalrami deep to the erector spinae muscle at midline.
Weobservedstaining of the subserratus tissue plane from T2 to T9andvariablestaining ofthe lateral branches of the intercostal nerves from T2 to T10 (see below) (Fig. 3A). Contrast was observed coursing medially, reaching the location of the exiting posterior
primary rami deep to the erector spinae muscle (Fig. 3B). Cranially, contrast staining deep to the rhomboid major and minor stopped at the levator scapula muscle in all specimens and was not observed beyond the serratus posterior superiorly, in proximity to the T2 level. Laterally and cranially, contrast extended to the clavipectoral fascia inside the axilla in 7 specimens (Fig. 3C), and inferiorly to the level of T10 around the insertion of the serratus posterior inferior muscle and origin of the external oblique muscle in 10 specimens (Fig. 3C).
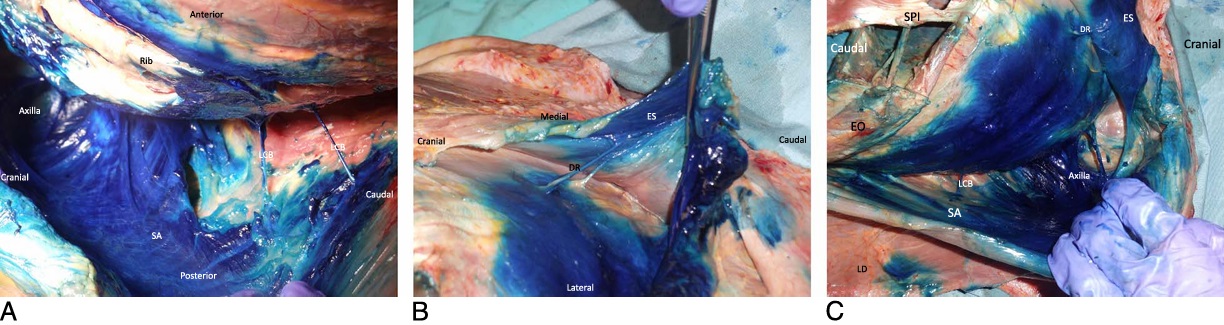
FIGURE 3. A, Cadaveric dissection showing spread of blue dye injectate in the subserratus tissue plane and staining of the lateral branches of the intercostal nerves. LCB indicates lateral cutaneous branches of intercostal nerves; SA, serratus anterior muscle. B, Cadaveric dissection showing spread ofblue dyeinjectateinthe tissue plane deep to the erectorspinae muscle (ES) staining the T6 and T7 dorsal rami of the spinal nerves (DR). C, Cadaveric dissection showing spread of blue dye injectate in the floor of the axilla, deep to the serratus anterior muscle (SA) up to the level of the second rib and dye reaching inferiorly to T10 around the insertion of the inferior portion of the serratus posterior muscle (SPI) and origin of the external oblique muscle (EO). LCB indicates lateral cutaneous branches of intercostal nerves; LD, latissimus dorsi muscle.
The lateral branches of the intercostal nerves stained con sistently across all specimens from T4 to T8. Ten specimens demonstrated consistent staining of T2 and T3. Nine specimens demonstrated consistent staining of T9 and T10 deep to the up per slips of the external oblique muscle (Fig. 4). Below the eighth thoracic level the lower 4 slips of the serratus anterior muscle interdigitate at their origins with the upper 5 slips of the external oblique muscle.25 Also below the eighth thoracic level, the serratus anterior muscle ends and this tissue plane be comes deep to the latissmus dorsi and the upper part of the external oblique muscle. In 8 specimens, faint staining of the tissue plane deep to the erector spinae muscles from T4 to T8 was observed and continued to the tissue plane posterior to the thoracic transverse processes. In 4 specimens, staining stopped at the lateral edge of the erector spinae, and no staining was observed deep to the erector spinae muscle (Cadaveric Clarification, Supplemental Digital Content 4, http://links.lww.com/AAP/A270).
We were able to identify the dorsal rami of the thoracic intercostal nerves of T6 and T7 as they emerge between the tips of adjacent transverse processes in only 2 cadavers (Fig. 3B). No specimens demonstrated intercostal nerve staining within the intercostal spaces either anteriorly or posteriorly. Staining was seen in the floor of the axilla in nine specimens, deep to the serratus anterior muscle up to the level of the second rib and posterior to the clavipectoral fascia.
In 2 of these 9 specimens, there was faint staining within the axilla and around the axillary artery. No staining was observed within the axilla in 3 specimens. We did not identify the lateral pectoral, medial pectoral, long thoracic, or thoracodorsal nerves
(summarized in Fig. 4). We did not observe any evidence of epidural or paravertebral spread.
This case series included 15 patients. Clinical data are summarized in Table 1. Four patients received single-shot blocks, and 11 patients received continuous catheters. Indications were
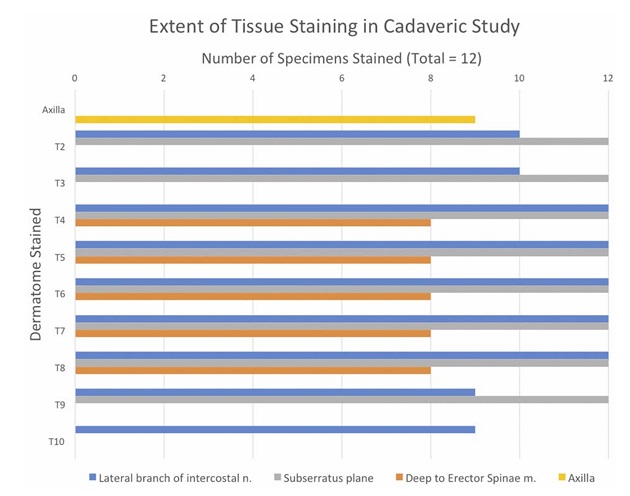
FIGURE 4. Histogram, the extent of tissue staining in cadaveric specimens. Nerve staining was consistently seen across all specimens from T4 to T8. Ten specimens demonstrated consistent staining of T2 and T3. Nine specimens demonstrated consistent staining of T9 and T10 deep to the upper slips of the external oblique muscle. Nine specimens demonstrated staining of the axilla.
either incisional or nonincisional pain of the chest wall, including rib fracture (2 patients), chest tube (2 patients), upper abdominal surgery (7 patients), cancer pain (1 patient), lung transplant (2 patients), and pneumonectomy (1 patient). The points of injection
were tailored to the anatomic location of pain, with rhomboid in tercostal injection points ranging from T3 to T6 and subserratus plane injection point ranging from T4 to T10 (Fig. 5, A and B). Dermatomalcoldsensorydeficitswere consistently achieved from T5 to T9, with the most cephalad coverage to T2 and the most caudal coverage to T12. The dermatomal coverage included the anterior hemithoraxextending from 4 cm lateral of the midline at the medial extent, laterally to the axilla, and on the posterior hemithorax extending to the midscapular line 4 cm medial to the posterior axillary line. Data for pain scores before the procedure, meanpain scores measured after block, and the dermatomal levels covered in patients are listed in Table 1. The average duration of
analgesia for single-shot blocks was 16 hours, and the average duration of catheter infusion was 3.6 days. No patients experienced any adverse reactions resulting from the blocks or catheters, in cluding pneumothorax, hypotension, urinary retention, upper
or lowerextremity weakness,or insertion site bleeding or infection (Detailed Patients Management, Supplemental Digital Con tent 8, http://links.lww.com/AAP/A274).
We demonstrate that injection in the tissue plane located be tween the rhomboid and intercostal muscles and then deep to the scapula and serratus anterior muscle targets the lateral cutaneous branches of the ventral rami of thoracic intercostal nerves. Spread
extends medially deep to the erector spinae tissue plane and superficial to the thoracic transverse processes at the point where the dorsal rami of the thoracic intercostal nerves emerge between the tips of adjacent transverse processes T3 to T9. In addition, we provide cadaveric evidence that the tissue plane deep to the erector spinae muscle, rhomboid muscles, serratus anterior muscles, latissimus dorsi, and the upper part of the external oblique muscle is continuous. To our knowledge, this has not been described as one continuous tissue plane. The RISS block leads to reproducible dermatomal analgesic coverage of the thorax upper abdomen and can be useful for cancer pain, postoperative pain after thoracotomy, chest tube associated pain, rib fracture pain, and upper abdominal incisional pain and as a supplement to a patchy thoracic epidural. We observed no extension to the intercostal nerves. The thoracodorsal and long thoracic nerves lie superficial to the serratus anterior muscle and should therefore be spared using the RISS block. In the cadaveric component of the study, we noted less consistent spread to the dorsal rami which is congruent with results of the clinical component of the study wherein the posterior mid line area was not blocked consistently in all patients. Given that this injection is made into an interfascial plane, the final needle location for injection can be varied, depending on desired dermatomal coverage. Specifically, the rhomboid inter costal plane injection can be made anywhere from approximately T3 to T6 (rhomboid major and minor muscles originate atthe C7 T5 spinous processes medially and course inferolaterally to insert at the medial border of the scapula at the T2 to T6 level), and the subserratus plane injection can be made anywhere from approxi mately T4 to T10 (serratus anterior muscle originates at the T1
T9 ribs and inserts along the medial border of the scapula). The rhomboidintercostal tissue plane overlaps with the subserratus tissue plane laterally and communicates from T1 to T5. We posit that the RISS combination block can be considered as an alternative approach to, or modification of, the serratus
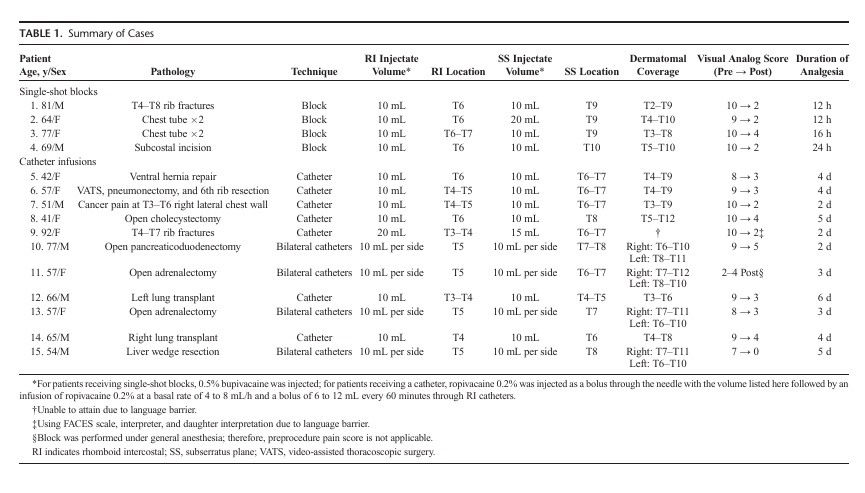
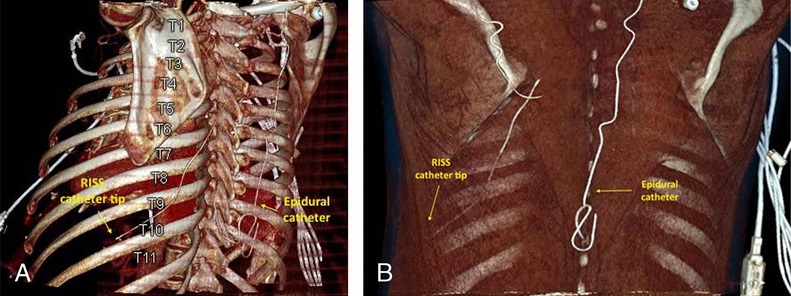
FIGURE5. A,Computed tomography with reconstructed 3-dimensiona limage demonstrating the RISS catheter tip located at the midaxillary line adjacent toT10.B,Computed tomography scan with reconstructed 3 dimensional image demonstrating catheter insertion point at the level of T7 at a point medial to the inferior edge of the scapula and deep to the latissimus dorsi and serratus anterior muscles. The tip of the catheter is positioned near the midaxillary line at approximately the T10 level. These images are of patient 5 from Table 1 who had RISS catheter placed for inadequate T8-9 epidural coverage.
anterior plane block and erector spinae plane block and can be used when those blocks are not feasible because of technical issues or proximity to the wound. Furthermore, the RISS block may offer some advantages over the pectoral nerves blocks and superficial serratus plane blocks. The site of injection for these blocks is close to the incision for many procedures, including breast surgery, thoracotomy, pneumonectomy, and chest tube placement. This creates technical challenges in performing post operative blocks as well as logistic challenges in placing catheters preoperatively because they may interfere with the sterile surgical field. The point of injection for RISS block is distant from most surgical incisions, and a catheter is unlikely to interfere with the surgical field. Another advantage of the RISS block over the serratus plane block is that it consistently blocks the lateral cutaneous branches of the intercostal nerves in the mid and lower thoracic dermatomes and thus can be utilized for upper abdominal surgery. Unlike the
superficial serratus plane block, the RISS block does not block the long thoracic nerve and thus should avoid potential winging of the scapula. The RISS block also has easily identifiable ultra sound landmarks (intercostal muscles and ribs), is amenable to
catheter insertion, and utilizes injection points that can be adjusted to match the coverage area to the site of the inciting pain. For the cadaveric injections, we selected 0.5% methylcellulose and india ink, as their physical characteristics are approximate to
that of local anesthetics. In addition, we used unembalmed fresh hu man cadavers at room temperature to maintain tissue elasticity as close as possible to live human tissue. All 12 cadaveric injections were performed at the same location, and contrast spread was uni form. In the clinical portion of the study, injection points were tai
lored to fit the dermatomal distribution of pain. This sample of 15 patients was not large enough to analyze for a correlation be tween injectate location and resultant dermatomal spread. None the less, the patient with the most cephalad injection point
(patient 12, Table 1) developed the most cephalad dermatomal coverage (T3–T6), whereas the patient with the most caudad injectate locations (patient 4, Table 1) developed the most caudad dermatomal coverage (T5–T10). Further study is warranted to evaluate the correlation between injectate or catheter location and resultant dermatomal coverage. Weconsider the2-pointinjection as a dissecting technique for the interfascial planes. Both in jections are not needed for all patients if the desired dermatomal coverage is small because the 2 tissue planes are continuous.
We recognize several limitations to both the cadaveric and patient case series. First, cadaveric evidence may not directly predict clinical outcomes; thus, cautious interpretation of the cadaveric results is warranted. Second, our cadaveric technique did notinvolve insertion of a catheter, aswasperformed inthe majority of the clinical cases presented. The clinical case series was a limited retrospective review. The RISS block has recognized disadvantages. First, there is inconsistent blockade of the axilla. Second, we did not observe analgesia in distributions of the anterior cutaneous branches of the intercostal nerves or the supraclavicular nerves, and the block did not cover the midline anteriorly or posteriorly. Third, the thoracodorsal nerve is not blocked, potentially limiting use as monotherapy for latissimus dorsi flap creation. Like other chest wall interfacial plane blocks, RISS block did not demonstrate staining of the intercostal nerves and therefore lacks visceral analgesic coverage, limiting its utility for visceral and neuropathic pain.26 Cadaveric studies provide detailed anatomic findings that are not possible in live subjects, but study in live subjects via a ran domized trial is needed, wherein comparison with paravertebral block or other interfascial plane blocks can assess outcomes and identify failure rates.
This initial description and evaluation of the RISS block demonstrates the concept of continuity between chest wall fascial planes with consistent spread of injectate to the lateral cutaneous branches of the T 4 toT 9 intercostal nervesin cadavers and consis
tent analgesia from the T5 to T8 dermatomes in a clinical case series, showing promise for this block in providing chest wall and upper abdominal wall analgesia.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of Dr Rahul
Renapurkar, Sections of Thoracic and Cardiovascular Imaging,
Imaging Institute, Cleveland Clinic, to the reconstructed com
puted tomography images presented in this article.
1. Forero M, Rajarathinam M, Adhikary S, Chin KJ. Continuous erector
spinae plane block for rescue analgesia in thoracotomy after epidural
failure: a case report. A A Case Rep. 2017;8:254–256.
2. Li NL, Yu BL, Hung CF. Paravertebral block plus thoracic wall block
versus paravertebral block alone for analgesia of modified radical
mastectomy: a retrospective cohort study. PLoS One. 2016;11:e0166227.
3. Blanco R,Parras T, McDonnell JG, Prats-Galino A.Serratus plane block: a
novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68:
1107–1113.
4. Daga V, Narayanan MK, Dedhia JD, Gaur P, Crick H, Gaur A. Cadaveric
feasibility study on the use of ultrasound contrast to assess spread of
injectate in the serratus anterior muscle plane. Saudi J Anaesth.2016;10:
198–201.
5. Hards M, Harada A, Neville I, et al. The effect of serratus plane block
performed under direct vision on postoperative pain in breast surgery.
JClinAnesth. 2016;34:427–431.
6. Ohgoshi Y, Yokozuka M, Terajima K. Serratus-intercostal plane block for
brest surgery [in Japanese]. Masui.2015;64:610–614.
7. Bhoi D, Pushparajan HK, Talawar P, Kumar A, Baidya DK. Serratus
anterior plane block for breast surgery in a morbidly obese patient.
J Clin Anesth. 2016;33:500–501.
8. Khemka R, Chakraborty A, Ahmed R, Datta T, Agarwal S.
Ultrasound-guided serratus anterior plane block in breast reconstruction
surgery. A A Case Rep. 2016;6:280–282.
9. Zocca JA, Chen GH, Puttanniah VG, Hung JC, Gulati A.
Ultrasound-guided serratus plane block for treatment of postmastectomy
pain syndromes in breast cancer patients: a case series. Pain Pract.2017;
17:141–146.
10. Piracha MM, Thorp SL, Puttanniah V, Gulati A. “A tale of two planes”:
deep versus superficial serratus plane block for postmastectomy pain
syndrome. Reg Anesth Pain Med. 2017;42:259–262.
11. Kwon WK, Choi JW, Kang JE, et al. Long thoracic nerve block in
video-assisted thoracoscopic wedge resection for pneumothorax. Anaesth
Intensive Care. 2012;40:773–779.
12. Broseta AM,ErrandoC,DeAndresJ,Diaz-CambroneroO,Ortega-Monzo
J. Serratus plane block: the regional analgesia technique for thoracoscopy?
Anaesthesia. 2015;70:1329–1330.
13. Kunhabdulla NP, Agarwal A, Gaur A, Gautam SK, Gupta R, Agarwal A.
Serratus anterior plane block for multiple rib fractures. Pain Physician.
2014;17:E553–E555.
14. Womack J, Varma MK. Serratus plane block for shoulder surgery.
Anaesthesia. 2014;69:395–396.
15. May L, Hillermann C, Patil S. Rib fracture management. BJA Education.
2016;16:26–32.
16. Moll V, Groff R, Budhrani G, Sathiyakumar A, McKenzie-Brown AM.
Bilateral serratus anterior plane block facilitates ventilator weaning in
patient with rib cage pain. Crit Care Med. 2016;44:561.
17. OkmenK,OkmenBM,UysalS.Serratus anterior plane (SAP) block used
for thoracotomy analgesia: a case report. Korean J Pain.2016;29:189–192.
18. Madabushi R, Tewari S, Gautam SK, Agarwal A, Agarwal A. Serratus
anterior plane block: a new analgesic technique for post-thoracotomy pain.
Pain Physician.2015;18:E421–E424.
19. Khalil AE, Abdallah NM, Bashandy GM, Kaddah TA. Ultrasound-guided
serratus anterior plane block versus thoracic epidural analgesia for
thoracotomy pain. J Cardiothorac Vasc Anesth. 2017;31:152–158.
20. Barbera C, Milito P, Punturieri M, Asti E, Bonavina L. Serratus anterior
plane block for hybrid transthoracic esophagectomy: a pilot study. JPain
Res.2017;10:73–77.
21. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae
plane block: a novel analgesic technique in thoracic neuropathic pain.
RegAnesthPainMed.2016;41:621–627.
22. Searle RD, Simpson MP, Simpson KH,MiltonR,Bennett MI. Can chronic
neuropathic pain following thoracic surgery be predicted during the
postoperative period? Interact Cardiovasc Thorac Surg. 2009;9:999–1002.
23. Elsharkawy H, Saifullah T, Kolli S, Drake R. Rhomboid intercostal block.
Anaesthesia. 2016;71:856–857.
24. Wilke J, Krause F, Vogt L, Banzer W. What is evidence-based about
myofascial chains: a systematic review. Arch Phys Med Rehabil.2016;97:
454–461.
25. NasuH,YamaguchiK,NimuraA,AkitaK.Ananatomicstudyofstructure
and innervation of the serratus anterior muscle. Surg Radiol Anat.2012;34:
921–928.
26. Mayes J, Davison E, Panahi P, et al. An anatomical evaluation of the
serratus anterior plane block. Anaesthesia. 2016;71:1064–1069.
