Abstract: Breast surgery is exceedingly common and may result in significant acute as well as chronic pain. Numerous options exist for the control of perioperative breast pain, including several newly described regional anesthesia techniques, but anesthesiologists have an insufficient understanding of the anatomy of the breast, the anatomic structures disrupted by the various breast surgeries, and the theoretical and experimental evidence supporting the use of the various analgesic options. In this article, we review the anatomy of the breast, common breast surgeries and their potential anatomic sources of pain, and analgesic techniques for managing perioperative pain. We per formed a systematic review of the evidence for these analgesic techniques, including intercostal block, epidural administration, paravertebral block, bra chial plexus block, and novel peripheral nerve blocks.
(Reg Anesth Pain Med 2017;42: 609–631)
Surgeries of the breast are among the most common operative procedures, and numerous options exist for perioperative anes thesia and analgesia that can affect acute perioperative pain, persistent pain, and potentially cancer recurrence. Patients who undergo breast surgery experience significant acute pain, but are also at risk of chronic pain. Up to 55% of postmastectomy patients experience chronic pain persisting for months to years.1–5 One of the best pre dictors of chronic pain following breast surgery is the amount of perioperative pain experienced by the patient.6–12 Although multiple options exist for control of perioperative breast surgery pain, including several newly described regional anesthesia techniques, there is insufficient understanding of the anatomy of the breast, the anatomic structures disrupted by the various breast surgeries, and the theoretical and experimental evidence supporting the use of the various analgesic options. In part I of this article, we review the anatomy of the breast and common breast surgeries, along with the potential anatomic sources of perioperative pain. We conclude with a discussion of the anatomic basis for different analgesic techniques. In part II, we present a systematic review of the evidence for the analgesic techniques including multimodal analgesia, local anesthetic infiltration, intercostal block, epidural administration, paravertebral block (PVB), brachial plexus block, and novel peripheral nerve blocks.
To determine the evidence base for perioperative breast analge sia techniques, we performed a literature search of the MEDLINE database via PubMed on November 5, 2016. The search combined terms for breast surgery, anesthesia, and analgesia. The PubMed search terms were ((“Analgesics” [MeSH] OR “analgesics” [all fields] OR “Analgesia” [MeSH] OR “analgesia” [all fields] OR “Anesthesia, Conduction” [MeSH] OR “nerve block” [all fields] OR “regional anesthesia” [all fields] OR “epidural” [all fields] OR “spinal anesthesia” [all fields] OR “neuraxial” [all fields] OR “general anesthesia” [all fields] OR “anaesthesia” [all fields] OR “anesthesia” [all fields] OR (“local” [all fields] AND “infiltration” [all fields])) AND (“Mammaplasty” [MeSH] OR “Mastectomy” [MeSH]OR“mammaplasty”[allfields] OR “mastectomy”[all fields] OR “breast surgery” [all fields] OR “chest wall” [all fields] OR “ax illa*” [all fields] OR “Breast Neoplasms/surgery”[MeSH] OR “breast cancer surgery” [all fields] OR “mammary” [all fields] OR “breast augmentation” [all fields] OR “axillary surgery” [all fields])). The search was not limited by date and excluded conference abstracts. The search was replicated in the EMBASE database for articles published in journals not indexed in MEDLINE.
Articles were included if they met the following inclusion criteria: (1) randomized controlled trial (RCT) design; (2) partici pants were humans at least 18 years of age undergoing elective surgery on the breast (not including biopsy); (3) published in En glish and full text available; (4) analgesic interventions were inter costal block, interpleural block, epidural block, PVB, or novel peripheral nerve blocks; (5) outcome measureswere postoperative analgesic consumption, postoperative pain scores, or duration of postoperative analgesia; and (6) minimum Jadad score of 2. Article titles and abstracts were screened, full-text articles were reviewed, and risk-of-bias assessments were performed by 2 authors indepen dently (R.M.J.I. and R.B.M.), with any discrepancies resolved through discussion. Risk of bias was assessed using the 5-point
scale described by Jadad et al.13 Articles using a study design other than RCT but meeting all other inclusion criteria were subsequently reviewed for findings not yet confirmed in RCTs.
The search strategy captured 5418 articles, of which 4407 were eliminated for failure to meet the inclusion criteria based on their titles and abstracts. Full-text review of the remaining 1011 articles eliminated an additional 965 articles that failed to meet the inclusion criteria, yielding a total of 46 articles (Fig. 1). Articles were organized according to intervention: 5 in tercostal block, 0 interpleural block, 5 epidural block, 31 PVB, 1 brachial plexus block, and 4 novel peripheral nerve block.
Part I: AnatomyoftheBreast,Surgical Disruption, and
the AnatomicBasis for Regional Analgesia Techniques
Innervation of the Breast and Superficial Tissues
Several distinct nerves innervate the breast and surrounding tissues. The majorityof the cutaneous sensation to the breast is de rived from the intercostal nerves. Upon exiting the intervertebral foramina, the thoracic spinal nerves divide into dorsal and ventral rami. The dorsal rami innervate the skin and muscles over the
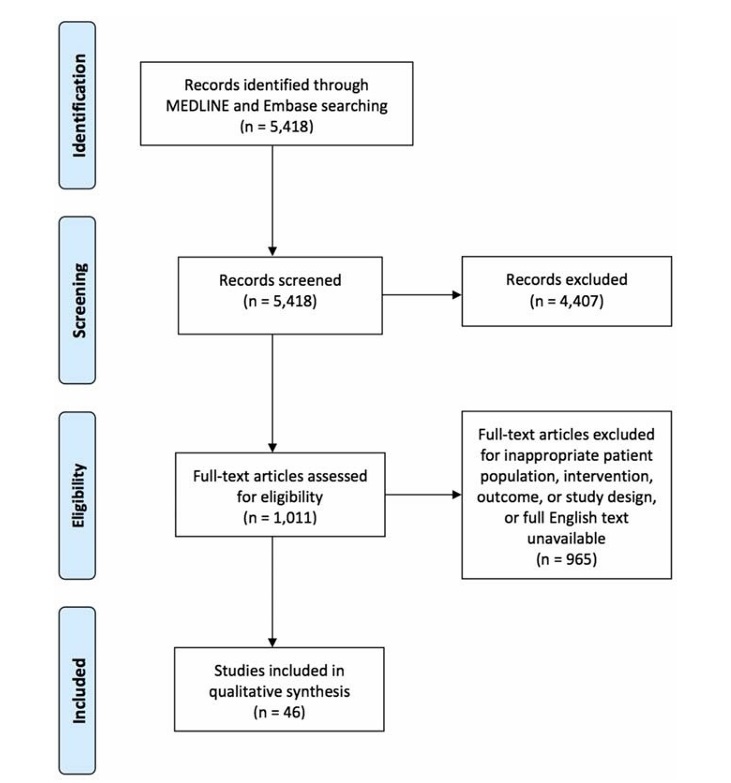
FIGURE 1. Flowchart of identified, screened, excluded, and analyzed studies.
medial back (Fig. 2). The ventral rami pass through the paravertebral space and become the intercostal nerves, which travel in the intercostal space just below the inferior border of the superior rib and are accompanied by anintercostal vein and artery. Much like the abdominal musculature, the intercostal region is composed of 3 muscle planes.14 From superficial to deep, the muscular planes are formed by the external intercostal muscle, the internal or intermediate intercostal muscle, and an innermost layer composed of the subcostal (posterior), innermost intercostal, and transversus thoracis (anterior) muscles. The intercostal nerves travel in the plane between the innermost layer and the internal in tercostal muscle (Fig. 2).15 Near the midpoint between the spine and sternum, at approximately the angle of the rib and midaxillary
line, a lateral cutaneous branch arises from the intercostal nerve and pierces the internal intercostal, external intercostal, and serratus anterior muscles (SAMs).15 The lateral cutaneous branches then divide into anterior and posterior divisions that pro vide cutaneous innervation to the lateral chest (Figs. 2–4). The continuation of the intercostal nerve terminates as an anterior cutaneous branch (ACB) by piercing the fascial extension of the external intercostal muscle close to the lateral edge of the sternum, providing cutaneous innervation to the medial chest and sternum (Figs. 2–4).15–17 In many texts, the anterior division of the lateral cutaneous branch is referred to as the anterior branch. We have used the term “anterior division” in order to avoid confusion with the terminal portion of the intercostal nerve, the ACB.
The breast is essentially a subcutaneous organ that receives innervation from anterior and lateral cutaneous branches of inter costal nerves, aswell as supraclavicular nerves. Published descriptions of the specific nerves involved and their courses vary significantly, likely because of both anatomic variability and differences in research methodology. The most commonly described pattern of innervation of the medial breast is by the ACBs of the T2 through T5 intercostal nerves with variable involvement of T1 and T6 and innervation of the lateral breast by the lateral cutaneous branches of the T2 through T5 intercostal nerves with variable involvement of T1, T6, and T7 (Figs. 3, 4).16–20 The first intercostal nerve rarely gives off a lateral cutaneous branch. Both
the lateral and anterior branches of different intercostal nerves fre quently communicate with each other throughout their course, producing a variable pattern of innervation that does not adhere to strict dermatomal segmentation.
The relationship of the cutaneous nerves of the breast to the underlying muscles is important as surgeons must avoid these nerves, and anesthesiologists seek to block them. After piercing
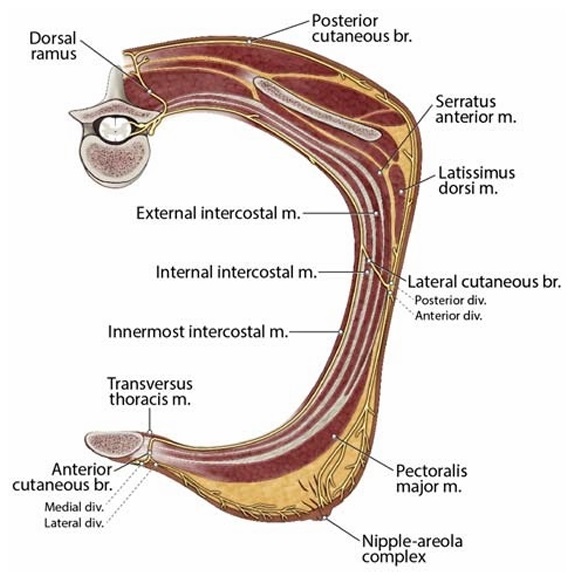
FIGURE 2. Course of the intercostal nerves. The ventral rami of the thoracic spinal nerves form the intercostal nerves, which travel in the intercostal space just below the inferior border of the superior rib. The intercostal region is composed of 3 muscle planes. From superficial to deep, the muscular planes are formed by the external
intercostal, the internal or intermediate intercostal muscle, and an innermost layer composed of the subcostal, innermost intercostal, and transversus thoracis muscles. The intercostal nerves travel in the plane between the innermost layer and the internal intercostal muscle. Near the midpoint between the spine and sternum, a lateral cutaneous branch arises from each intercostal nerve and pierces the internal intercostal, external intercostal, and SAMs. The lateral cutaneous branches then divide into anterior and posterior divisions that provide cutaneous innervation to the
lateral chest. The continuation of each intercostal nerve terminates as an ACB by piercing the fascial extension of the external intercostal muscleclose to the lateral edge ofthe sternum,providing cutaneous innervation to the medial chest and sternum. Br indicates branch; div, division; m, muscle.
the internal and external intercostal muscles, the lateral cutaneous branches of the intercostal nerves penetrate through the slips of origin of the SAM. The anterior divisions of these nerves course over the lateral edge of the pectoralis major (PM) muscle to reach the cutaneous tissue of the chest (Figs. 3, 4). The T4 and T5 lateral cutaneous branches may also give rise to a deep branch that pierces the PM muscle before reaching the breast.17
The nipple-areola complex (NAC) is innervated by both an terior and lateral branches of the intercostal nerves T3 through T4, with variable contribution from T2 and T5. The exact innervation of the NAC is still controversial because of numerous anatomic variations and the difficulty in dissecting this area.21 The most common descriptions of NAC innervation detail a comingling of the terminal branches of the anterior divisions of the lateral cuta neous branches of the T4 and T5 intercostal nerves and the termi nal branches of the ACBs.17–19,22
Special consideration should begiven tothe course of the lat eral cutaneous branch arising from the T2 intercostal nerve, termed the intercostobrachial nerve. As with the other lateral cu taneous branches, this nerve branches off the intercostal nerve around the angle of the rib. The lateral aspect of the T2 rib lies in the axilla. After piercing the intercostal and SAMs, the majority of the lateral cutaneous branch of T2 travels laterally along the floor of the base of the axilla to reach the upper medial arm (Fig. 5). The intercostobrachial nerve provides cutaneous innervation to the axillary tail of the breast, the axilla, and the medial up per arm. The extrathoracic anatomy of this nerve is highly
variable. It may receive contributions from other intercostal branches (T1, T3, and even T4) and can have a variety of anasto moses with branches of the brachial plexus, including the medial antebrachial cutaneous nerve, posterior cutaneous nerve of the
forearm, and rarely with the pectoral nerves.23–25 This nerve is often implicated in postmastectomy pain, particularly after axillary dis section or lymph node sampling.26,27 Its variable anatomy may ac count for the conflicting reports of post–nerve injury symptoms.
In addition to the innervation of the breast tissue and skin from the intercostal nerves, a small portion of the superior breast skin may be innervated by the supraclavicular nerves, although this description has been disputed.16,17,28 These nerves originate from the superficial cervical plexus and eventually travel in the subcutaneous tissue to pass over the clavicle and reach the superior aspect of the breast (Figs. 3, 4).
Innervation of the Chest Wall
Although the cutaneous innervation of the breast is derived from the intercostal nerves with a small contribution from the supraclavicular nerves, the brachial plexus supplies the innervation to the muscles of the chest wall (other than the intercostal muscles, which derive their innervation from the intercostal nerves). The majority of the breast tissue is immediately anterior to the pectoralis muscles. The upper portion of the PM muscle is supplied by the lateral pectoral nerve (LPN), whereas the medial
pectoral nerve (MPN) innervates the pectoralis minor (Pm) muscle and the lower portion of PM.29,30 These nerves arise from the brachial plexus at variable locations and take a variable course

FIGURE 3. Innervation of the breast. Medially, the ACBs of the intercostal nerves can be seen piercing the PM near the sternum to innervate the medial breast. The supraclavicular nerves cross the clavicle and innervate the skin inferior to the clavicle and potentially a portion of the superior pole of the breast. The lateral cutaneous branches of the intercostal nerves divide into anterior andposterior divisions, which pierce the SAM. The anterior divisions provide innervation to the lateral breast. Also depicted are the posterior divisions, which can be seen entering the subcutaneous tissue to innervate the lateral chest wall. The lateral cutaneous branch of T2 forms the intercostobrachial nerve, which innervates the axilla and the medial upper arm. Br indicates branch; div, division;m,muscle;n,nerve.
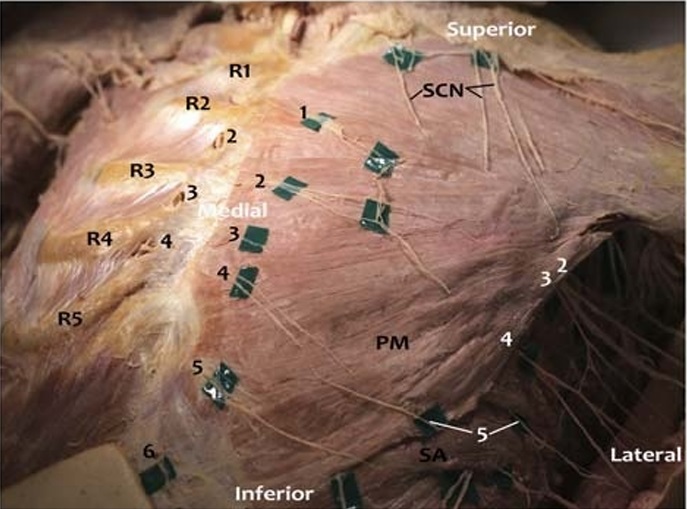
FIGURE 4. Photograph of anterior body wall nerves.The breast and subcutaneous tissue has been removed from this female donor, while preserving the nerves. Anterior cutaneous branches (black numbers) of the intercostal nerves emerge from the intercostal spaces and travel inferolaterally before piercing the PM muscle. On
the donor right, the PM has been reflected. The ACBs on the donor right are visible exiting inferior to the rib through the internal intercostal muscle and external intercostal fascia, which has been partially removed to demonstrate the nerve exit point. This exit point is slightly superior to the ACBs exiting through the PM on the donor left. Fine branches of the ACBs were once connected to each other, to the supraclavicular nerves, and to the anterior divisions of lateral cutaneous branches. The supraclavicular nerves can be seen traveling caudally to innervate the superior chest. These nerves may reachthe superiorpole of the breast tissue, which overlays the second or third rib. On the lateral chest wall, the divisions of the lateral cutaneous branches (LCB, white numbers) of the intercostal nerves can be seen piercing the SAM. The anterior division of the fifth LCB is visible, whereas other anterior divisions entering the lateral breast were lost during removal of the breast tissue. The nerves that can be seen extending laterally are the posterior divisions. In this dissection, we noted the anterior and posterior divisions of the fifth LCB exited through the serratus anterior at different locations. R1–R5 indicates first to fifth ribs; SA, serratus anterior; SCN, supraclavicular nerves.
to the pectoralis muscles (Fig. 6). The LPN is derived from the
C5–7 nerve roots and arises most frequently from the anterior di
vision of the upper trunk of the brachial plexus or from the lateral
cord, from which it derives its name.31 The MPN is derived from
the C7–T1 nerve roots and usually arises from the medial cord.
Both nerves depart the axilla to travel medially toward the
pectoralis muscles.29,30,32–35 The LPN crosses the lateral and su
perior border of the Pm muscle to enter the plane between the
PM and Pm muscles, usually alongside the pectoral artery
(Figs. 6, 7).36 This fascial plane and artery are often used as sono
graphic landmarks to locate the LPN. The LPN provides numer
ous branches that penetrate the deep surface of the PM muscle
and supply the innervation to the superior and medial aspect of
the muscle. The MPN usually travels deep (posterior) to the Pm
muscle, supplying its innervation, before coursing anteriorly to
supply innervation to the inferior portion of the PM. The MPN
may pierce the Pm muscle or emerge from beneath the inferior
edge of the muscle to reach the PM, or both (Figs. 6, 7). Although
these nerves do not innervate the subcutaneous tissue of the
breast, they still play an important role in breast surgical pain. Dis
ruption, stretching, or spasm of the pectoral muscles or associated
fascia can be a significant source of myofascial pain after breast surgery.37,38 Although often labeled simply as motor nerves, they
have been described to carry both nociceptive and proprioceptive
fibers.39 In addition to proprioception, all of the motor nerves to
the chest wall carry postganglionic fibers from the cervical and
thoracic ganglion, which may be another mechanism for commu
nication of pain.40 Aside from injury to the tissues supplied by the
nerves, traction, radiation, or other direct nerve injury may play a
role in postsurgical neuropathic pain.40
Twoother important nerves in the region of the axilla and lat
eral chest wall are the long thoracic and thoracodorsal nerves
(TDNs), both of which originate from the brachial plexus. The
long thoracic nerve (LTN) arises from the C5–7nerveroots.Upon
reaching the infraclavicular region, it runs along the lateral chest
wall superficial to the SAM, which it innervates (Fig. 6). Disrup
tion of the SAMduringbreastreconstruction(makinga pocketfor
an implant) can result in myofascial pain to the chest wall radiat
ing to the subscapular region.38
The TDN is derived from the C6–8 nerve roots and arises
from the posterior cord of the brachial plexus. It exits the posterior
wall of the axilla to travel along the anterior and lateral portion of
the latissimus dorsi muscle in close proximity to the subscapular
artery, where it innervates the latissimus dorsi muscle (Figs. 6, 7).
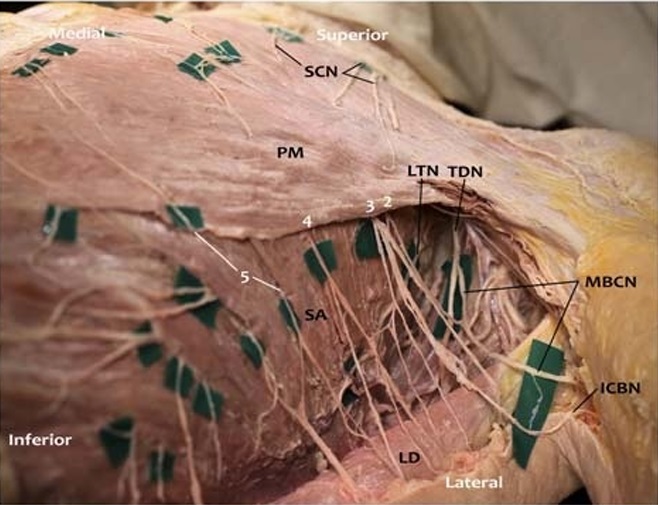
FIGURE 5. Photograph of left lateral chest wall and axilla with subcutaneous tissue removed. In this photograph, the subcutaneous tissue and breast have been removed, while preserving the nerves in this female cadaver. The dissection was extended to expose the axilla. Lateral cutaneous branches (LCB, white numbers) of the intercostal nerves emerge between the leaflets of the serratus anterior (SA) muscle. Most of the anterior divisions of the LCBs were removed along with the removal ofthe breast tissue. Posterior divisions of the LCBs travel posterolaterally to innervate the skin of the lateral body wall. In this specimen, the posterior division of the second LCB emerges deep to the PM and anastomoses with the third LCB to form the intercostobrachial
nerve (ICBN). The posterior division of the second LCB also anastomoses with the medial brachial cutaneous nerve (MBCN) to innervate the axilla and upper arm. This is a common anatomical occurrence. Also shown are the TDN, which innervates latissimus dorsi (LD), and the LTN, which innervates the SA muscle. The anterior and posterior divisions of the fifth LCB emerge separately throughtheSA, with theanterior division coming around the lateral border of PM to innervate the breast. In this specimen, separate exit points of the divisions of the lateral cutaneous branches of the
intercostal nerves through the serratus anterior were observed. The anterior divisions of the fourth and fifth LCBs (and likely the second and third) anastomosed with the ACBs of the intercostal nerves. Anastomoses with the supraclavicular nerves were also
observed.
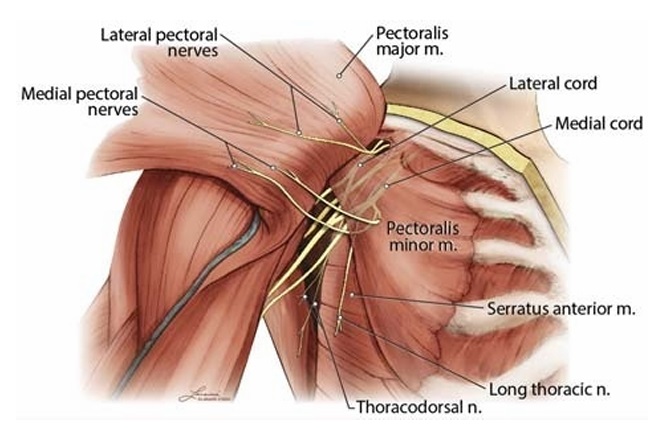
FIGURE 6. Diagram of pectoral nerves. In this diagram, the PM muscle was reflected laterally to demonstrate the pectoral nerves arising from the brachial plexus. The LPN is depicted arising from the lateral cord of the brachial plexus and innervates the PM. The MPN is show narising from the medial cord. Some of the branches of the MPN pierce the Pm muscle, which it innervates, to reach the caudal aspect of the PM. Another branch of the medical pectoralnerve courses caudal to the edge of Pm to reach the PM. Both courses of the MPN branche sare common. Also depicted is the LTN innervating the SAM and the TDN innervating the latissimus dorsi muscle. Both nerves arise from the brachial plexus. Not shown are the cutaneous branches of the intercostal nerves. N indicates nerve; m, muscle.
Similar to the LTN, injury to this nerve has been implicated in
postmastectomy pain.38,40
Breast Surgery and Tissue Disruption
Knowledge of the precise anatomic location of tissue disruption for each type of breast surgery is imperative in developing a perioperative analgesic plan. Operations involving the breast can differ substantially with regard to the tissues that are removed or compromised. Breast cancer procedures are discussed first.
Excisional Breast Surgery
Lumpectomy involves excision of a wedge of subcutaneous breast tissue. A partial (segmental or quadrantectomy) mastectomy is performed if more breast tissue warrants removal. This procedure is performed for tumors that are too large for lumpec
tomy, for patients who cannot tolerate radiation, or if more than 1 distinct area of the breast is involved. As with a lumpectomy, only subcutaneous breast tissue is removed.41 Depending on whether surgery is performed medial or lateral to the nipple, the anterior or lateral cutaneous branches of the intercostal nerves (respectively) will contribute to the innervation of the operative area. Atotal (simple) mastectomy involves removing the entire subcutaneous breast tissue and varying amounts of overlying skin.
The underlying fascia of the PM muscleisnotdisrupted.42 Similar to lumpectomies and partial mastectomies, the intercostal nerves are responsible for the innervation to the surgical area. When de veloping a plan for perioperative analgesia, it is important to deter mine if an axillary dissection or sentinel node biopsy will be performed in conjunction with a partial or total mastectomy. Surgery in the axilla is in the territory of the intercostobrachial nerve (lateral cutaneous branch of T2), which may require separate blockade, depending on the chosen analgesic approach. Aradical mastectomy is a moreextensive breast cancer oper ation involving removal of the entire breast, nipple, axillary lymph nodes, and pectoralis muscles. More commonly, a mastectomy
and sentinel node biopsy are performed, or alternatively, a modified radical mastectomy, which includes a mastectomy and an axillary dissection but preserves the pectoralis muscles, is per formed.43 The borders of dissection extend superiorly to the clavicle, medially to the sternum, inferiorly to the most caudal extent of breast tissue (on the costal margin below the inframammary fold), and laterally in the axilla to the border of the latissimus dorsi. The fascia of the PM muscle forms the deep margin of the dissec
tion and is removed during the procedure, which may constitute a source of postoperative myofascial pain.44 The dissection also fre quently involves removal of breast tissue or lymph nodes that re side between the inferior edge of the PM muscle and the Pm muscle. This is important as the MPN can be injured, resulting
in partial denervation of the PM muscle.32,33 In addition, manipu lation and stretching of the pectoralis muscles may be another source of perioperative myofascial pain. One or 2 drains may be placed through separate inferior-lateral incisions and can be addi
tional sources of pain below the dermatomes associated with the breast. Mastectomy requires general anesthesia or an advanced re gional block with sedation. In contrast to pain from simple mastectomy, brachial plexus–derived nerves (lateral and medial pectoral, thoracodorsal, long thoracic) can also contribute to perioperative modified radical mastectomy pain (Fig. 7).
Reconstructive Breast Surgery
Because of the disfiguring nature of breast cancer surgery, surgical reconstruction to restore a natural breast appearance is frequently performed in conjunction with breast cancer proce dures.45 In reconstructions with an implant, a tissue expander is
usually placed beneath the PM muscleandanterior tothe Pm. Laterally, the SAM may be elevated to cover the inferolateral pole of the implant.46 The inflatable bladder is expanded over days to weeks to slowly stretch the overlying PM muscle, fascia, and skin. In a second operation, the temporary expander is replaced by a long-term implant. It is important to identify whether a tissue ex pander will be placed after a total mastectomy. Unlike total mas tectomy without a tissue expander, this procedure will involve blunt dissection of a pocket for the expander between the pectoral
muscles. Creation of the pocket can be a source of additional periprocedure and chronic pain due to direct disruption of the pectoral nerves or lateral cutaneous branches of the intercostal nerves
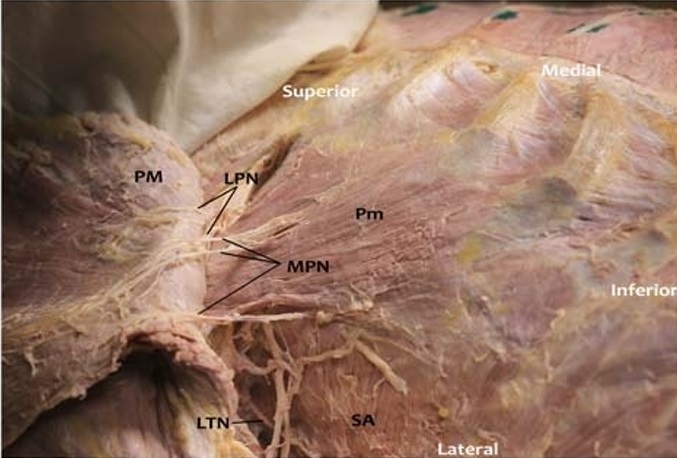
FIGURE 7. Photograph of pectoral nerves. In this photograph, the subcutaneous tissue and breast have been removed, while preserving the nerves in this female cadaver. The PM muscle is reflected laterally to show branches of the LPN onitsdeepsurface. Two branches of the MPN pierce the Pm muscle to innervate the inferior third of PM, and 1 branch of the MPN circumscribes the lateral border of the Pm (accompanied by an artery and vein) to innervate the most inferior fibers of the PM. Posteriorly, the LTN is demonstrated innervating the SA muscle.
or due to stretching or injury to the fascia of the serratus anterior or pectoral muscles. Women who under go breast cancer surgery with immediate reconstruction have a higher prevalence of chronic pain when compared with women who undergo mastectomy with out reconstruction (49% and 31%, respectively).12 An important
consideration in developing a perioperative analgesic plan for breast surgery with reconstruction is the involvement of not only the intercostal nerves, but also the pectoral nerves and possibly the LTN (Fig. 7).
With implant reconstruction, a sheet of mesh or acellular der mal matrix is often used to provide internal support to the implant and to extend the lower border of the PM muscle. This allows the muscle to be held in place against the chest wall at the desired inframammary fold and provides an additional source of coverage material for the lower pole of the implant. This material is sewn directly to the chest wall, with sutures placed in rib periosteum or deep chest wall fascia, often creating a significant source of
postoperative discomfort. Analgesia is targeted to the level of the sixth and seventh intercostal spaces (at the level of the inframammary fold) to address pain from these sutures.
Another type of reconstruction, either delayed or immediate, involves a free or pedicle flap to recreate breast volume.45 The most common procedures include transverse rectus abdominis myocutaneous flaps, either performed as a pedicled or free flap; deep inferior epigastric artery perforator as a free flap; and latissimus dorsi myocutaneous flaps as a pedicled flap. When developing a perioperative analgesic plan, the donor site for the flap must be considered in addition to the breast surgery itself. For example, a patient undergoing total mastectomy with immediate pedicled transverse rectus abdominis myocutaneous flap reconstruction mayexperience more postoperative pain from the abdominalwall donor site than from the mastectomy site.47,48 This is due to the resection of the rectus muscle and closure ofthe rectus sheath(primarily or with synthetic mesh).
When a traditional latissimus dor si flap is used for breast re construction, an ellipse of skin is harvested from the back over the lower thoracic region to act as the donor site. The entire latissimus muscle is then elevated from a pocket that extends subcutaneously from the mid–lumbar region inferiorly to the tip of the scapula su
periorly and from the paraspinous muscle fascia medially to the posterior axillary line laterally. The flap is passed through a tunnel near the axilla to the anterior chest. Any indwelling anesthetic catheter would need to cover the entire hemiback to the posterior
midline on the side of the flap. It is also important to note that this muscle is innervated by the TDN, a branch of the brachial plexus.
Thoracic Epidural
The most common levels for placement of epidural injec
tions or catheters for breast analgesia is T3–5. Similar to intercos
tal blocks, thoracic epidural anesthesia (TEA) without cervical
spread would not block the branches of the brachial or cervical
plexus that may contribute to perioperative breast surgery pain.
Paravertebral Block
The paravertebral space can be accessed to block the thoracic
spinal nerves as they exit the intervertebral foramina. Local anes
thetic deposited in this space can spread multiple levels superior
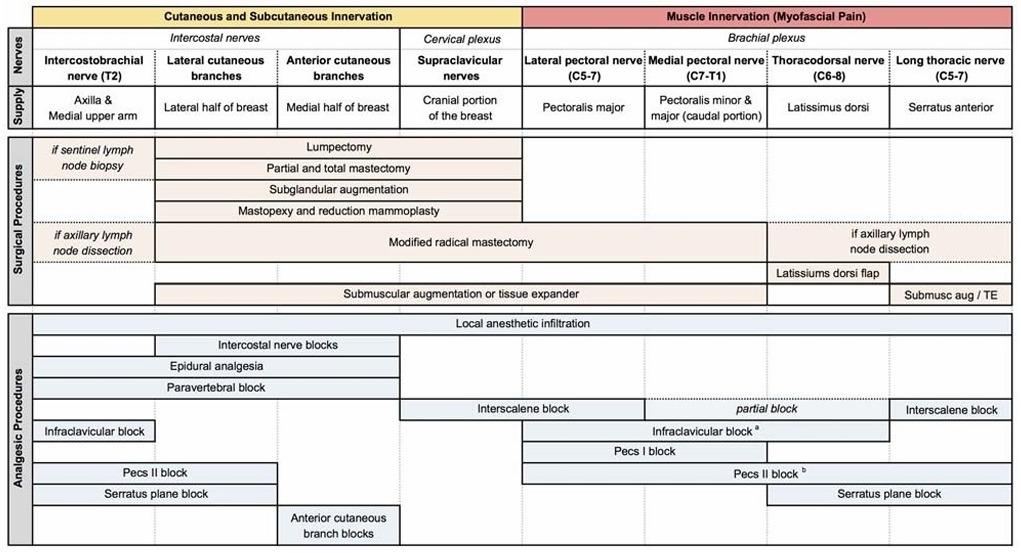
FIGURE 8. Summary of chest wall innervation, breast surgeries, and analgesic procedures. The innervation of the chest wall is summarized, along with the various breast surgeries organized by the corresponding nerves involved. The regional analgesic procedures and the nerves anesthetized by each approach are depicted. aInfraclavicular block may also anesthetize the LTN and lateral cutaneous branches of the intercostal nerves through spread along the lateral chest wall. As the extent and frequency of this spread remain unproven, block of these nerves is not depicted. b Pecs II block may spare the LTN when using the modified technique of injecting local anesthetic deep to the SAM. TE indicates tissue expander.
and/or inferior, as well as into the intercostal space laterally, the con tralateral paravertebral space, and the epidural space medially.53 This technique generally results in ipsilateral blockade of somatic and sympathetic nerves and can serve as the sole anesthetic for breast surgery, as long as blockade of the supraclavicular nerves, pectoral nerves, or other brachial plexus branches is not required (similar to TEA).
Brachial Plexus and Novel Peripheral Nerve Blocks
Alternative regional techniques have been proposed in an ef fort to provide equivalent or improved analgesia with a lower risk of adverse events and greater suitability for outpatient surgery (see part II for the evidence base behind claims of efficacy and safety for each technique). Initial efforts were aimed at blocking some or all of the peripheral nerves of brachial plexus origin that provide sensory innervation to the breast: LPN, MPN, LTN, and TDN. Subsequent efforts have aimed to expand the utility of these novel blocks by simultaneously anesthetizing the cutaneous branches of the intercostal nerves.
Brachial plexus blocks. The LPN, MPN, and TDN typically arise from the anterior division of the upper trunk or lateral cord, medial cord, and posterior cord, respectively. Thus, infraclavicular brachial plexus block at the level of the cords would be expected to block these brachial plexus components of breast analgesia, and possibly the LTN as it courses along the lateral chest wall. In contrast, interscalene block at the level of the C5–7 nerve roots would be expected to block the LTN, but only partial blockade of
the LPN, MPN, and TDN because of absence of reliable C8 and T1nerve root block. It is important to note that interscalene block is not expected to provide axillary analgesia, because sensory innervation of the axilla is derived primarily from the intercostobrachial nerve (T2 intercostal nerve). Because of its proximity to the axilla, infraclavicular block typically does result in block of the intercostobrachial nerve and axilla. Bigeleisen and Wilson 54 dem onstrated 77% and 87% incidence of intercostobrachial nerve block with 10 mL volume infraclavicular block via a medial and lateral approach, respectively. Although anatomically possible, the extent to which infraclavicular block provides local anesthetic spread along the chest wall to anesthetize the lateral cutaneous branches of the intercostal nerves other than the intercostobrachial nerve, and thus provide breast analgesia, has not been adequately studied. With the possible exception of infraclavicular blocks, brachial plexus blocks alone will not anesthetize the thoracic intercostal nerves supplying the breast and would not be sufficient for complete breast analgesia.
Pecs I block. Novel blocks have recently been introduced in an effort to anesthetize key nerves derived from the brachial plexus, avoid blocking the brachial plexus nerves that innervate the arm, and block the cutaneous branches of the intercostal
nerves. Blanco55 was the first to describe a novel ultrasound guided interfascial plane block, the Pecs I block, targeting the LPN and MPN via an injection between the PM and Pm muscles (eg, 0.25% bupivacaine 0.4 mL/kg). Distribution of local anesthetic in this plane is expected to anesthetize the LPN as it courses between the PM and Pm muscles and the MPN as it courses anteriorly through or at the lateral margin of the Pm
muscle, with the goal of reducing postoperative muscle spasm and myofascial pain from the pectoralis muscles (eg, surgeries involving the pectoral muscles, including tissue expander and subpectoral prostheses placement).55 Note that evidence is needed to support this theoretical mechanism of chest wall an algesia (see part II).
Pecs II block. In order to expand the utilityof interfascial pe ripheral nerve blocks for breast surgery, Blanco et al56 proposed a modification of the Pecs I block, called the Pecs II block. This block is performed with ultrasound guidance at the level of ribs 2–4. Pecs II block consists of 2 injections, one deep injection be tween the Pm muscle and the SAM with 20 mL bupivacaine 0.25% and one superficial injection identical to the Pecs I block (between the PM and Pm muscles) with 10 mL bupivacaine 0.25%. Both injections can be made via one skin puncture site and oftenvia 1 needle pass. The terminology of this block (named the Pecs II block or modified Pecs I block by Blanco) has led to some confusion as some authors have erroneously used “Pecs II” to describe the deep injection alone.
The addition of the deep injection (between the Pm muscle and the SAM) targets 3 distinct nerve groups: the anterior divi sions of the lateral cutaneous branches of the intercostal nerves that pierce the external intercostal muscle and SAM at approxi mately the midaxillary line, the LTN that courses along the super ficial surface of the SAM, and the TDN that courses along the deep surface of the latissimus dorsi muscle. Of note, in an effort to improve interfascial spread and spare the LTN, Pérez et al 57 proposed a modification of the Pecs II block, wherein the deep injection is performed deep to the SAM rather than superficial to the SAM. Sparing of the LTN would allow for postoperative assessment given the risk of surgical injury to this nerve during axillary dissection.57
The ACBs of the intercostal nerves (terminal portion of the intercostal nerves exiting near the sternum) would not be expected to be anesthetized with a Pecs II block unless local anesthetic were to diffuse deep to the SAM and the external and internal intercos tal muscles to reach the intercostal nerve. Thus, a Pecs II block alone would be expected to leave sensory innervation of the me dial breast intact.
Serratus plane block.Blancoetal58 described another vari ation of the Pecs blocks, termed the serratus plane block (SPB), with the goal of providing extended intercostal nerve coverage. This block is performed more distal and lateral than Pecs II block,
overlying the fifth rib at the midaxillary line. The latissimus dorsi muscle is visualized with ultrasound overlying the SAM. Identifi cation of the thoracodorsal artery can verify the plane between the 2 muscles. Local anesthetic (eg, bupivacaine 0.125% 0.4 mL/kg)
can be deposited either superficial or deep to the SAM, in an at tempt to provide sensory block of the T2–T9 dermatomes.58
De la Torre et al59 and Alfaro–de la Torre and Fajardo-Pérez60 have developed a nearly identical block termed the serratus intercostal fascial block that involves deposition of local anes thetic deep to the SAM,between the SAM and external intercostal muscle. It is important to understand that SPB replaces only the deep injection of Pecs II block (between the Pm muscle and SAM), necessitating addition of a Pecs I block to cover the LPN (eg, for subpectoral device implantation).57
Similar to the Pecs II block, the SPB likely blocks only the lateral cutaneous branches of the intercostal nerves, thus failing to anesthetize the medial breast. It is unclear if the local anesthetic diffuses through the SAM and external interocostal and internal
intercostal muscles to reach the intercostal nerve proximal to the origin of the lateral cutaneous branch. Closer proximity to the in tercostal nerves is one of the theoretical advantages of injection deep to the SAM, but this remains insufficiently studied.
Blocks of the ACBs of the intercostal nerves. Inorder to target the ACBs of the intercostal nerves, de la Torre et al59 de scribed the pectointercostal fascial block (PIFB). This ultrasound guided block is performed at the medial aspect of the breast, 2 to 3 cmlateral to the sternal border at the level of the fourth rib. The PM muscle is visualized superficial to the external intercostal muscle, andlocal anesthetic is deposited between these 2 muscles.
In a similar approach,Ueshima and Kitamura 61 described the transversus thoracis muscle plane (TTP) block. This block is per formed in the parasternal location; however, the local anesthetic is deposited in a deeper interfascial plane between the transversus thoracis muscle (deep) and the internal inter costal muscle (superficial).61 Potential anatomic disadvantages of this approach are the very close proximity to the pleura (be
cause TTP is very thin)15 and the internal thoracic artery (because it travels in the same plane between the transverse thoracis muscle and the internal intercostal muscle approximately 1 cm lateral to the sternum).62 The extent of local anesthetic spread and subsequent analgesia following PIFB and TTP block require further study.
It is important to recognize the anatomic limitations of the in dividual interfascial approaches. None of these approaches are ex pected to block the supraclavicular nerves that may supply a small portion of the superior breast.63 Only the PIFB and TTP block anes thetize the ACBs of the intercostal nerves that supply the medial as pect of the breast. Thus, combinations of blocks are necessary to provide complete analgesia for many surgical procedures.59
With a sophisticated understanding of the anatomy relevant to breast surgery and analgesia, combinations of various blocks will likely provide postoperative analgesia and even surgical anes the sia for a wide variety of operations (Fig. 8). Before adopting
these promising blocks, a review of the evidence for their efficacy and safety is warranted.
Part II: Systematic Review of the Evidence for
Regional Analgesia Techniques for
Breast Surgery
Intercostal Nerve Blocks
Five studies evaluating intercostal nerve block with a median Jadad score of only 2 (range, 2–3) were included in the review. Of these 5 studies, 3 demonstrated improved analgesia in patients receiving intercostal blocks (Table 1).64–66 The role of intercostal
nerve blocks in contemporary practice is questionable, given the mixed results, the risks associated with these multilevel blocks, and the presence of alternative peripheral approaches including local anesthetic infiltration and the novel interfascial peripheral
nerve blocks. High-quality studies to directly compare intercostal nerve blocks to these alternative techniques, with attention to both analgesic benefit and adverse effects, are needed to clarify the utility of intercostal blocks for patients undergoing breast surgery.
Epidural Administration
Five studies evaluating TEA with a median Jadad score of 3 were included in the review. Of these 5 studies, 2 studies 71,72 uti lized continuous postoperative local anesthetic infusion, 2 studies 69,70 utilized epidural anesthesia in the operating room only, and 1 study 68 utilized epidural morphine alone (Table 2). Three studies 69,70,72 demonstrated effective surgical anesthesia with epidural block for modified radical mastectomy or mastectomy. All 5 studies demonstrated analgesic benefit with an epidural technique, although only 2 utilized a double-blind design. In the studies utilizing continuous postoperative epidural infusion, analgesic benefit persisted until epidural discontinuation (second postoperative day).71,72 In addition to reductions in pain scores and analgesic consumption, epidural anesthesia resulted in shorter hospital stay,71 faster achievement of postanes the sia care unit discharge readiness,72 and improved patient satisfaction.69,70,72 Although the evidence supports the ability of epidural technique to provide both surgical anesthesia and postoperative analgesia, concerns regarding adverse events and logistic constraints have prevented this technique from becoming common practice for breast surgery.
Paravertebral Block
Thirty-one studies of PVB with a median Jadad score of 3 (range, 2–5) were included in the review. Of these, 10 studies73–81 used a single injection at 1 level, 11 studies83–86,88–91 utilized a single injection at multiple levels, and 10 studies9,94–102 utilized continuous infusions (Table 3). Single-level injections were performed at T2–T4, and multilevel injections were performed at 3 to 7 levels ranging from C7–T7. Twenty-three studies compared PVB to alternative analgesic approaches (ie, general anesthesia, intravenous opioids, local infiltration). All but 1 of these 24 studies101 identified analgesic benefit of PVB as indicated by pain scores, analgesic consumption, or time to
first analgesic. It should be noted that only 4 of the 23 positive studies used a double-blind design.
The optimal dosing strategy with PVB for breast surgery (single injection, single injection with additives, or continuous in fusion) remains unclear. The reported duration of analgesic bene fit for patients with single-injection PVB varied between studies, with evidence of analgesia as late as postoperative day 3.80
Additional outcomes were reported in several studies. Seven studies reported an improvement in postoperative nausea and vomiting,73,75,78,83,84,90,92 and 2 reported shortened length of hospital stay.84,90 In addition, 4 studies (only 2 of which were double-blind) demonstrated an improvement in pain incidence or characteristics at 1 to 12 postoperative months.9,76,101,103
In summary, the literature supports PVB as an effective peri operative analgesic technique for breast surgery. Paravertebral block can also provide surgical anesthesia and may decrease nau sea and vomiting, hospital stay, and chronic postsurgical pain. The use of paravertebral catheters has not reliably been demonstrated to be superior to a single-injection technique at 1 or multiple levels. Similar to epidurals, the safety of PVBs for outpatient sur gery is a concern, given the trend toward outpatient performance of breast surgeries. Outpatient breast surgery with ambulatory
paravertebral catheters has been described, but its analgesic bene fit has not been demonstrated.97,100 Finally, further studies com paring PVB to local anesthetic infiltration are needed.
Brachial Plexus and Novel Peripheral Nerve Blocks
Brachial Plexus Blocks
Onebrachial plexus block study104 with a Jadad score of 3 was included in the review (Table 4). Kaya et al104 demon strated analgesic benefit of relatively large-volume (30 mL) interscalene block alone for modified radical mastectomy. In addition, Sundarathitiet al70 included an interscalene block as a sup plement to TEA, but the study design prevents an assessment of the specific impact of the interscalene block (Table 2). In summary, although 1 study lends some support to the theoretical benefit of brachial plexus blocks, the undesirable upper-extremity block that results has prevented the incorporation of these approaches into clinical practice.
Interfascial Plane Blocks
No RCTs of Pecs I block were identified in our review. Four Pecs II block studies with a median Jadad score of 3 were in cluded in our review105–108 (Table 4). Bashandy and Abbas106 investigated Pecs II block versus no block for modified radical mastectomy in an observer-blinded study. They demonstrated reduced pain scores in the first 24 hours, reduced opioid consumption in the first 12 hours, less nausea and vomiting, less sedation, and shorter postanesthesia care unit and hospital stay in patients receiving Pecs II block. In an unblinded study, Wahba and Kamal105 compared Pecs II block to single-injection, 1-level T4 paravertebral for modified radical mastectomy under
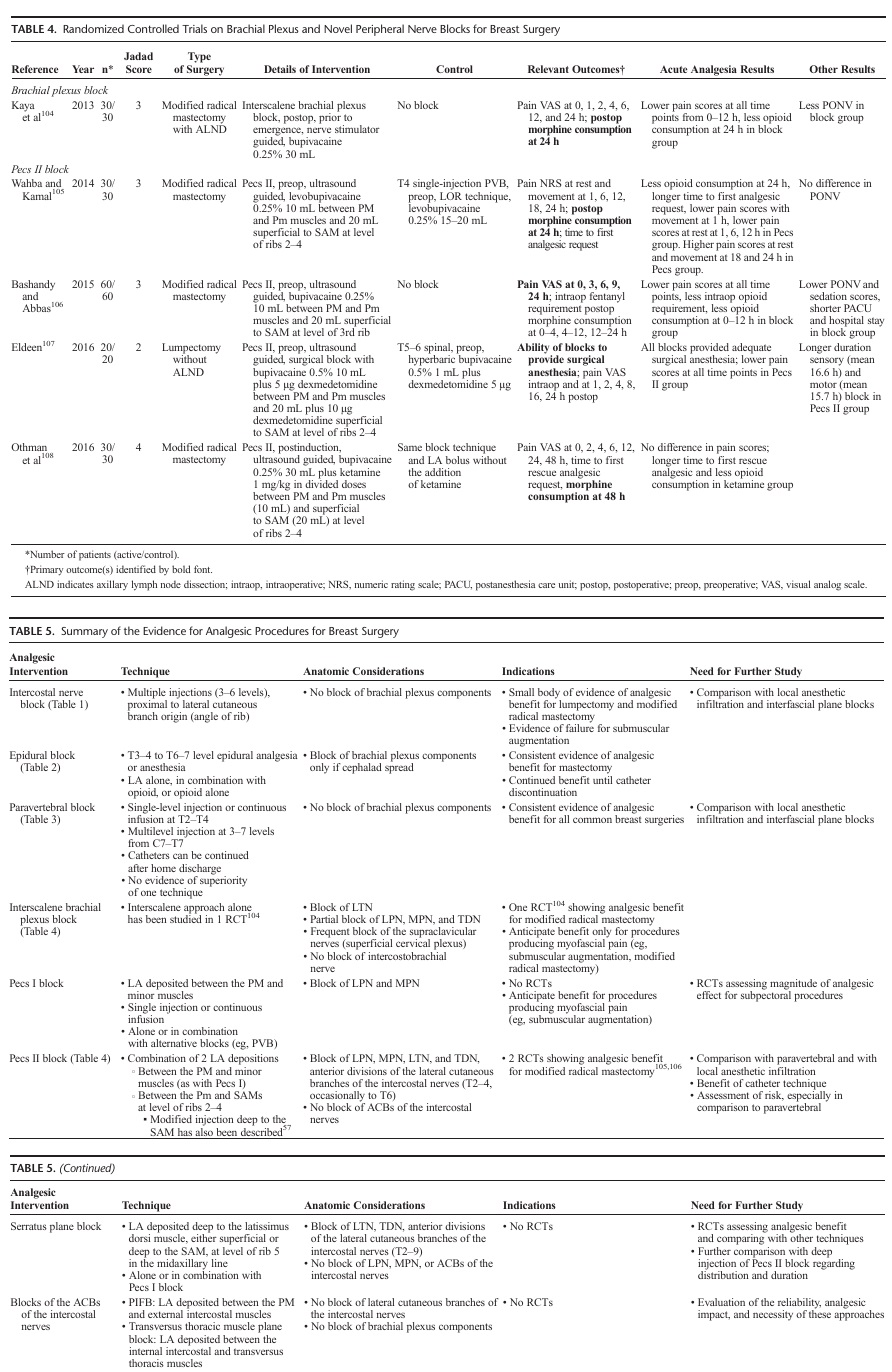
general anesthesia. They demonstrated reduced analgesic consumption at 24 hours, longer time to first analgesic request, and some reduction in pain scores in the first 12 hours with Pecs II block. Despite the use of levobupivacaine in both blocks, pain scores were higher at 16 and 24 hours in the Pecs II block group, suggesting shorter duration of effect with Pecs II than PVB despite improved early analgesia. Eldeen 107 demonstrated that Pecs II blocks can provide effective surgical anesthesia for lumpectomy without axillary lymph node dissection and a sensory block extending to a mean of 16.6 hours when using bupivacaine 0.5% combined with low dose dexmedetomidine.
No RCTs o fSPB, PIFB, or TTP block were identified in our review.
The biggest impact of interfascial peripheral nerve blocks could be their relative ease to perform and potentially low risk profile. Because of the peripheral nature of these blocks, sympathetic blockade is not expected, risk of serious bleeding is likely low, and, as with other ultrasound-guided interfascial blocks, performance of the block in both awake and anesthetized patients may be safe. Although the novel interfascial blocks hold promise, there is a clear paucity of high-quality evidence supporting the analgesic efficacy and addressing the safety of these approaches. Random ized controlled trials comparing the various technical ap proaches to one another and comparing the interfascial blocks to alternative techniques (especially local anesthetic infiltration and PVB) are needed.
Acute pain following breast surgery is common, and numerous options exist for perioperative analgesia. An under standing of the anatomy of the breast and the anatomic structures disrupted by various surgical procedures will aid in selecting the appropriate perioperative analgesic technique and evaluating new techniques as they are described in the literature. We summarize the innervation, surgical procedures,and analgesic procedures in Figure 8 and the evidence for each of the analgesic procedures in Table 5. Both epidural and PVBs have been shown to provide effective analgesia for breast surgery. Paravertebral block has consistently been demonstrated to enhance analgesia while improving addi tional aspects of postoperative recovery, but further RCTs comparing PVB directly to local anesthetic infiltration are needed. Novel interfascial peripheral nerve blocks show promise as they may be easier to perform, may decrease risk, may be more suitable for outpatient procedures, and may even provide more complete analgesia by blocking both in tercostal and brachial plexus–derived nerves. Although enticing, these assertions remain unproven. Randomized trials are needed to determine the safety and efficacy of the newer peripheral blocks, especially as compared with alternative analgesic techniques.
ACKNOWLEDGMENTS
The authors thank Anna Getselman, executive director,
Health Sciences Library, Columbia University Medical Cen
ter, for her significant contributions to the literature review.
1. Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The
postmastectomy pain syndrome: an epidemiological study on the
prevalence of chronic pain after surgery for breast cancer. Br J Cancer.
2008;99:604–610.
2. Cheville AL, Tchou J. Barriers to rehabilitation following surgery for
primary breast cancer. JSurgOncol. 2007;95:409–418.
3. Roth RS, Lowery JC, Davis J, Wilkins EG. Persistent pain following
postmastectomy breast reconstruction: long-term effects of type and
timing of surgery. Ann Plast Surg. 2007;58:371–376.
4. Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain
in breast cancer survivors: analysis of clinical, demographic, and
psychosocial factors. J Pain. 2013;14:1185–1195.
5. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H.
Prevalence of and factors associated with persistent pain following breast
cancer surgery. JAMA. 2009;302:1985–1992.
6. Cho AR, KwonJY, Kim KH, et al. The effects of anesthetics on chronic
pain after breast cancer surgery. Anesth Analg. 2013;116:685–693.
7. Bokhari FN, McMillan DE, McClement S, Daeninck PJ. Pilot study of a
survey to identify the prevalence of and risk factors for chronic
neuropathic pain following breast cancer surgery. Oncol Nurs Forum.
2012;39:E141–E149.
8. Fassoulaki A, Melemeni A, Staikou C, Triga A, Sarantopoulos C. Acute
postoperative pain predicts chronic pain and long-term analgesic
requirements after breast surgery for cancer. Acta Anaesthesiol Belg.
2008;59:241–248.
9. IohomG,AbdallaH,O’BrienJ, etal.Theassociations between severityof
early postoperative pain, chronic postsurgical pain and plasma
concentration of stable nitric oxide products after breast surgery. Anesth
Analg. 2006;103:995–1000.
10. Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain
following breast cancer surgery: a prospective study. J Pain.2006;7:
626–634.
11. Andersen KG, Duriaud HM, Jensen HE, KromanN, Kehlet H.Predictive
factors for the development of persistent pain after breast cancer surgery.
Pain. 2015;156:2413–2422.
12. Wallace MS, Wallace AM, Lee J, Dobke MK. Pain after breast surgery: a
survey of 282 women. Pain. 1996;66:195–205.
13. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of
randomized clinical trials: is blinding necessary? Control Clin Trials.
1996;17:1–12.
14. Siddiqi MA, Mullick AN. On the anatomy of intercostal spaces in man
and certain other mammals. JAnat. 1935;69:350–355.
15. Davies F, Gladstone RJ, Stibbe EP. The anatomy of the intercostal nerves.
JAnat. 1932;66:323–333.
16. Jaspars JJ, Posma AN, van Immerseel AA, Gittenberger-de Groot AC.
The cutaneous innervation of the female breast and nipple-areola
complex: implications for surgery. Br J Plast Surg. 1997;50:249–259.
17. SarhadiNS,ShawDunnJ,LeeFD,SoutarDS.Ananatomicalstudyofthe
nerve supply of the breast, including the nipple and areola. Br J Plast
Surg. 1996;49:156–164.
18. Cooper A. On the Anatomy of the Breast. London: Longman, Orme,
Green, Brown, and Longmans; 1840.
19. Craig RD, Sykes PA. Nipple sensitivity following reduction
mammaplasty. Br J Plast Surg. 1970;23:165–172.
20. Michelle le Roux C, Kiil BJ, Pan WR, Rozen WM, Ashton MW.
Preserving the neurovascular supply in the Hall-Findlay superomedial
pedicle breast reduction: an anatomical study. J Plast Reconstr Aesthet
Surg. 2010;63:655–662.
21. Schlenz I, Kuzbari R, Gruber H, Holle J. The sensitivity of the nipple
areola complex: an anatomic study. Plast Reconstr Surg. 2000;105:
905–909.
22. Riccio CA, Zeiderman MR, Chowdhry S, et al. Plastic surgery of the
breast: keeping the nipple sensitive. Eplasty. 2015;15:e28.
23. Loukas M, Louis RG Jr, Fogg QA, Hallner B, Gupta AA. An unusual
innervation of pectoralis minor and major muscles from a branch of the
intercostobrachial nerve. Clin Anat. 2006;19:347–349.
24. Loukas M, Hullett J, Louis RG Jr, Holdman S, Holdman D. The gross
anatomy of the extrathoracic course of the intercostobrachial nerve. Clin
Anat. 2006;19:106–111.
25. O’Rourke MG, Tang TS, Allison SI, Wood W. The anatomy of the
extrathoracic intercostobrachial nerve. Aust N Z J Surg.1999;69:860–864.
26. Kwekkeboom K. Postmastectomy pain syndromes. Cancer Nurs.1996;
19:37–43.
27. Vecht CJ, van de Brand HJ, Wajer OJ. Post-axillary dissection pain in
breast cancer due to a lesion of the intercostobrachial nerve. Pain.1989;
38:171–176.
28. Corriveau S, Jacobs JS. Macromastia in adolescence. Clin Plast Surg.
1990;17:151–160.
29. Macchi V, Tiengo C, Porzionato A, et al. Medial and lateral pectoral
nerves: course and branches. Clin Anat. 2007;20:157–162.
30. Porzionato A, Macchi V, Stecco C, et al. Surgical anatomy of the pectoral
nerves and the pectoral musculature. Clin Anat. 2012;25:559–575.
31. AradE,LiZ,SitzmanTJ,AgurAM,ClarkeHM.Anatomicsitesoforigin
of the suprascapular and lateral pectoral nerves within the brachial plexus.
Plast Reconstr Surg. 2014;133:20e–27e.
32. Hoffman GW, Elliott LF. The anatomy of the pectoral nerves and its
significance to the general and plastic surgeon. Ann Surg. 1987;205:
504–507.
33. Moosman DA. Anatomy of the pectoral nerves and their preservation in
modified mastectomy. Am J Surg. 1980;139:883–886.
34. LoukasM,LouisRGJr,FitzsimmonsJ,Colborn G.Thesurgical anatomy
of the ansa pectoralis. Clin Anat.2006;19:685–693.
35. Tubbs RS, Jones VL, Loukas M, et al. Anatomy and landmarks for
branches of the brachial plexus: a vade mecum. Surg Radiol Anat.2010;
32:261–270.
36. Bremner-Smith AT, Unwin AJ, Williams WW. Sensory pathways in the
spinal accessory nerve. J Bone Joint Surg Br. 1999;81:226–228.
37. Layeeque R, Hochberg J, Siegel E, et al. Botulinum toxin infiltration for
pain control after mastectomy and expander reconstruction. Ann Surg.
2004;240:608–613.
38. HuangTT.Breastandsubscapular painfollowingsubmuscular placement
of breast prostheses. Plast Reconstr Surg. 1990;86:275–280.
39. Mense S. Nociception from skeletal muscle in relation to clinical muscle
pain. Pain. 1993;54:241–289.
40. Wallace AM, Wallace MS. Postmastectomy and postthoracotomy pain.
Anesthesiol Clin North Am. 1997;15:353–370.
41. Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to
partial mastectomy: an overview of volume-displacement techniques.
Lancet Oncol. 2005;6:145–157.
42. Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin
sparing mastectomy and immediate reconstruction in 657 breasts.
AnnSurgOncol. 2012;19:3402–3409.
43. Loukas M, Tubbs RS, Mirzayan N, Shirak M, Steinberg A, Shoja MM.
The history of mastectomy. Am Surg. 2011;77:566–571.
44. Vadivelu N, Schreck M, Lopez J, Kodumudi G, Narayan D. Pain after
mastectomy and breast reconstruction. Am Surg. 2008;74:285–296.
45. Cordeiro PG. Breast reconstruction after surgery for breast cancer. NEngl
JMed. 2008;359:1590–1601.
46. Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with
tissue expander/implant breast reconstruction: part I. A prospective
analysis of early complications. Plast Reconstr Surg. 2006;118:825–831.
47. Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site
complications and functional outcomes in free muscle-sparing TRAM
flap and free DIEP flap breast reconstruction. Plast Reconstr Surg.2006;
117:737–746.
48. Kroll SS, Sharma S, Koutz C, et al. Postoperative morphine
requirements of free TRAM and DIEP flaps. Plast Reconstr Surg.
2001;107:338–341.
49. Ghaderi B, Hoenig JM, DadoD, AngelatsJ, Vandevender D. Incidence of
intercostobrachial nerve injury after transaxillary breast augmentation.
Aesthet Surg J. 2002;22:26–32.
50. Hidalgo DA, Pusic AL. The role of methocarbamol and intercostal nerve
blocks for pain management in breast augmentation. Aesthet Surg J.2005;
25:571–575.
51. von Sperling ML, Hoimyr H, Finnerup K, Jensen TS, Finnerup NB.
Persistent pain and sensory changes following cosmetic breast
augmentation. Eur J Pain. 2011;15:328–332.
52. Kopacz DJT, GE. Intercostal blocks for thoracic and abdominal surgery.
Tech Reg Anesth Pain Manag. 1998;2:25–29.
53. Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95:
771–780.
54. Bigeleisen P, Wilson M. A comparison of two techniques for ultrasound
guided infraclavicular block. Br J Anaesth. 2006;96:502–507.
55. Blanco R. The ‘Pecs block’: a novel technique for providing analgesia
after breast surgery. Anaesthesia. 2011;66:847–848.
56. Blanco R, Fajardo M, Maldonado TP. Ultrasound description of Pecs II
(modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol
Reanim. 2012:1–6.
57. Pérez MF, Duany O, de la Torre PA. Redefining Pecs blocks for
postmastectomy analgesia. Reg Anesth Pain Med. 2015;40:729–730.
58. Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block:
a novel ultrasound-guided thoracic wall nerve block. Anaesthesia.2013;
68:1107–1113.
59. De la Torre PA, García PD, Alvarez SL, Miguel FJ, Pérez MF. A novel
ultrasound-guided block: a promising alternative for breast analgesia.
Aesthet Surg J. 2014;34:198–200.
60. Alfaro–de la Torre P, Fajardo-Pérez M. Thoracic paravertebral block and
its effects on chronic pain and health-related quality of life after modified
radical mastectomy. Reg Anesth Pain Med. 2015;40:177–178.
61. Ueshima H, Kitamura A. Blocking of multiple anterior branches of
intercostal nerves (Th2–6) using a transversus thoracic muscle plane
block. RegAnesthPainMed. 2015;40:388.
62. Henriquez-PinoJA,GomesWJ,PratesJC,BuffoloE.Surgicalanatomyof
the internal thoracic artery. Ann Thorac Surg. 1997;64:1041–1045.
63. Sopena-Zubiria LA, Cuellar-Martinez A, Galan Gutierrez JC, Fernandez
Mere LA. Reply to the article entitled ultrasound description of Pecs II
(modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol
Reanim. 2013;60:295.
64. Atanassoff PG, Alon E, Pasch T, Ziegler WH, Gautschi K. Intercostal
nerve block for minor breast surgery. Reg Anesth.1991;16:23–27.
65. Atanassoff PG, Alon E, Weiss BM. Intercostal nerve block for
lumpectomy: superior postoperative pain relief with bupivacaine. JClin
Anesth. 1994;6:47–51.
66. Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. Regional block
and mexiletine: the effect on pain after cancer breast surgery. Reg Anesth
Pain Med. 2001;26:223–228.
67. Nasr MW, Habre SB, Jabbour H, Baradhi A, El Asmar Z. A randomized
controlled trial of postoperative pain control after subpectoral breast
augmentation using intercostal nerve block versus bupivacaine pectoralis
major infiltration: a pilot study. J Plast Reconstr Aesthet Surg.2015;68:
e83–e84.
68. Aida S, Baba H, Yamakura T, Taga K, Fukuda S, Shimoji K. The
effectiveness of preemptive analgesia varies according to the type of
surgery: a randomized, double-blind study. Anesth Analg.1999;89:
711–716.
69. YehCC,YuJC, WuCT,HoST,ChangTM,WongCS.Thoracicepidural
anesthesia for painrelief and postoperation recovery with modified radical
mastectomy. World J Surg. 1999;23:256–260.
70. Sundarathiti P, Pasutharnchat K, Kongdan Y, Suranutkarin PE. Thoracic
epidural anesthesia (TEA) with 0.2% ropivacaine in combination with
ipsilateral brachial plexus block (BPB) for modified radical mastectomy
(MRM). J Med Assoc Thai. 2005;88:513–520.
71. Correll DJ, Viscusi ER, Grunwald Z, Moore JH Jr. Epidural analgesia
compared with intravenous morphine patient-controlled analgesia:
postoperative outcome measures after mastectomy with immediate
TRAMflap breast reconstruction. RegAnesthPainMed.2001;26:
444–449.
72. Doss NW, Ipe J, Crimi T, et al. Continuous thoracic epidural anesthesia
with 0.2% ropivacaine versus general anesthesia for perioperative
management of modified radical mastectomy. Anesth Analg.2001;92:
1552–1557.
73. Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E.
Single-injection paravertebral block compared to general anaesthesia in
breast surgery. Acta Anaesthesiol Scand. 1999;43:770–774.
74. Terheggen MA, Wille F, Borel Rinkes IH, Ionescu TI, Knape JT.
Paravertebral blockade for minor breast surgery. Anesth Analg.2002;94:
355–359.
75. Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ.
Single-injection paravertebral block before general anesthesia enhances
analgesia after breast cancer surgery with and without associated lymph
node biopsy. Anesth Analg. 2004;99:1837–1843.
76. Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional
paravertebral block reduces the prevalence of chronic pain after breast
surgery. Anesth Analg. 2006;103:703–708.
77. HuraG,KnapikP,MisiolekH,KrakusA,KarpeJ.Sensoryblockadeafter
thoracic paravertebral injection of ropivacaine or bupivacaine. Eur J
Anaesthesiol. 2006;23:658–664.
78. Sidiropoulou T, Buonomo O, Fabbi E, et al. A prospective comparison of
continuous wound infiltration with ropivacaine versus single-injection
paravertebral block after modified radical mastectomy. Anesth Analg.
2008;106:997–1001.
79. ArunakulP,RuksaA.Generalanesthesiawiththoracicparavertebralblock
for modified radical mastectomy. JMedAssocThai. 2010;93(suppl 7):
S149–S153.
80. Gardiner S, RudkinG,Cooter R,FieldJ, Bond M.Paravertebral blockade
for day-case breast augmentation: a randomized clinical trial. Anesth
Analg. 2012;115:1053–1059.
81. Bhuvaneswari V, Wig J, Mathew PJ, Singh G. Post-operative pain and
analgesic requirements after paravertebral block for mastectomy: a
randomized controlled trial of different concentrations of bupivacaine and
fentanyl. Indian J Anaesth.2012;56:34–39.
82. Hassan ME, Mahran E. Effect of adding magnesium sulphate to
bupivacaine on the clinical profile of ultrasound-guided thoracic
paravertebral block in patients undergoing modified radical mastectomy.
Eg JAnaesth.2015;31:23–27.
83. Klein SM, BerghA,Steele SM, Georgiade GS, Greengrass RA. Thoracic
paravertebral block for breast surgery. Anesth Analg. 2000;90:
1402–1405.
84. Naja MZ, Ziade MF, Lönnqvist PA. Nerve-stimulator guided
paravertebral blockade vs. general anaesthesia for breast surgery: a
prospective randomized trial. Eur J Anaesthesiol. 2003;20:
897–903.
85. Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic
paravertebral block for breast cancer surgery: a randomized double-blind
study. Anesth Analg. 2007;105:1848–1851.
86. Boughey JC, Goravanchi F, Parris RN, et al. Prospective randomized trial
of paravertebral block for patients undergoing breast cancer surgery. Am J
Surg. 2009;198:720–725.
87. Omar AM, Mansour MA, Abdelwahab HH, et al. Role of ketamine and
tramadol as adjuncts to bupivacaine 0.5% in paravertebral block for breast
surgery: a randomized double-blind study. Eg J Anaesth.2011;27:
101–105.
88. DasS,Bhattacharya P, Mandal MC, Mukhopadhyay S, BasuSR, Mandol
BK. Multiple-injection thoracic paravertebral block as an alternative to
general anaesthesia for elective breast surgeries: a randomised controlled
trial. Indian J Anaesth.2012;56:27–33.
89. Naja ZM, Ziade FM, El-Rajab MA, Naccash N, Ayoubi JM. Guided
paravertebral blocks with versus without clonidine for women undergoing
breast surgery: a prospective double-blinded randomized study. Anesth
Analg. 2013;117:252–258.
90. Abdallah FW, Morgan PJ, Cil T, et al. Ultrasound-guided multilevel
paravertebral blocks and total intravenous anesthesia improve the quality
of recovery after ambulatory breast tumor resection. Anesthesiology.
2014;120:703–713.
91. Mohamed SA, Fares KM, Mohamed AA, Alieldin NH.
Dexmedetomidine as an adjunctive analgesic with bupivacaine in
paravertebral analgesia for breast cancer surgery. Pain Physician.2014;
17:E589–E598.
92. Fallatah S, Mousa W. Multiplelevels paravertebral block versus morphine
patient-controlled analgesia for postoperative analgesia following breast
cancer surgery with unilateral lumpectomy, and axillary lymph nodes
dissection. Saudi J Anaesth.2016;10:13–17.
93. Wolf O, Clemens MW, Purugganan RV, et al. A prospective, randomized,
controlled trial of paravertebral block versus general anesthesia alone for
prosthetic breast reconstruction. Plast Reconstr Surg. 2016;137:
660e–666e.
94. Buggy DJ, Kerin MJ. Paravertebral analgesia with levobupivacaine
increases postoperative flap tissue oxygen tension after immediate
latissimus dorsi breast reconstruction compared with intravenous opioid
analgesia. Anesthesiology. 2004;100:375–380.
95. Burlacu CL, Frizelle HP, Moriarty DC, Buggy DJ. Fentanyl and clonidine
as adjunctive analgesics with levobupivacaine in paravertebral analgesia
for breast surgery. Anaesthesia. 2006;61:932–937.
96. McElwain J, Freir NM, Burlacu CL, Moriarty DC, Sessler DI, Buggy DJ.
The feasibility of patient-controlled paravertebral analgesia for major
breast cancer surgery: a prospective, randomized, double-blind
comparison of two regimens. Anesth Analg. 2008;107:665–668.
97. Buckenmaier CC 3rd, Kwon KH, Howard RS, et al. Double-blinded,
placebo-controlled, prospective randomized trial evaluating the efficacy of
paravertebral block with and without continuous paravertebral block
analgesia in outpatient breast cancer surgery. Pain Med.2010;11:
790–799.
98. Deegan CA, Murray D, Doran P, et al. Anesthetic technique and the
cytokine and matrix metalloproteinase response to primary breast cancer
surgery. Reg Anesth Pain Med. 2010;35:490–495.
99. Abdel-halim JMK.Continuous thoracicparavertebral block: an adjunct to
general anaesthesia in major breast surgery. Eg JAnaesth.2011;27:83–87.
100. Ilfeld BM, Madison SJ, Suresh PJ, et al. Treatment of postmastectomy
pain with ambulatory continuous paravertebral nerve blocks: a
randomized, triple-masked, placebo-controlled study. Reg Anesth Pain
Med. 2014;39:89–96.
101. KarmakarMK,SamyW,LiJW,etal. Thoracicparavertebral blockand its
effects on chronic pain and health-related quality of life after modified
radical mastectomy. RegAnesthPainMed. 2014;39:289–298.
102. Wu J, Buggy D, Fleischmann E, et al. Thoracic paravertebral regional
anesthesia improves analgesia after breast cancer surgery: a randomized
controlled multicentre clinical trial. Can J Anaesth. 2015;62:241–251.
103. Ilfeld BM, Madison SJ, Suresh PJ, et al. Persistent postmastectomy pain
and pain-related physical and emotional functioning with and without a
continuous paravertebral nerve block: a prospective 1-year follow-up
assessment of a randomized, triple-masked, placebo-controlled study.
Ann Surg Oncol. 2015;22:2017–2025.
104. Kaya M, Oguz G, Senel G, Kadiogullari N. Postoperative analgesia after
modified radical mastectomy: the efficacy of interscalene brachial plexus
block. JAnesth. 2013;27:862–867.
105. Wahba SS, Kamal SM. Thoracic paravertebral block versus pectoral
nerve block for analgesia after breast surgery. Eg J Anaesth. 2014;30:
129–135.
106. Bashandy GM, Abbas DN. Pectoral nerves i and ii blocks in multimodal
analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth
Pain Med.2015;40:68–74.
107. Eldeen HMS. Ultrasound guided pectoral nerve blockade versus thoracic
spinal blockade for conservative breast surgery in cancer breast: a
randomized controlled trial. Eg J Anaesth.2016;32:29–35.
108. OthmanAH,El-RahmanAM,ElSherifF.Efficacyandsafetyofketamine
added to local anesthetic in modified pectoral block for management of
postoperative pain in patients undergoing modified radical mastectomy.
Pain Physician. 2016;19:485–494.
