Introduction: Patients undergoing minimally invasive cardiac surgery have the potential for significant pain from the thoracotomy site. We report the successful use of pectoral nerve block types I and II (Pecs I and II) as rescue analgesia in a patient undergoing minimally invasive mitral valve repair.
Case Report: Inthis case, a 78-year-old man, with no history of chronic pain, underwent mitral valve repair via right anterior thoracotomy for severe mitral regurgitation. After extubation, he complained of 10/10 pain at the incision site that was minimally responsive to intravenous opioids. He required supplemental oxygen because of poor pulmonary mechanics, with shallow breathing and splinting due to pain, and subsequent intensive care unit readmission. Ultrasound-guided Pecs I and II blocks were per formed on the right side with 30 mL of 0.2% ropivacaine with 1:400,000 epinephrine. The blocks resulted in near-complete chest wall analgesia and improved pulmonary mechanics for approximately 24 hours. After
the single-injection blocks regressed, a second set of blocks was performed with 266 mg of liposomal bupivacaine mixed with bupivacaine. This second set of blocks provided extended analgesia for an additional 48 hours. Thepatient wasweanedrapidly from supplemental oxygen after the blocks because of improved analgesia.
Conclusions: Pectoral nerve blocks have been described in the setting of breast surgery to provide chest wall analgesia. We report the first successful use of Pecs blocks to provide effective chest wall analgesia for a patient un dergoing minimally invasive cardiac surgery with thoracotomy. We believe that these blocks may provide an important nonopioid option for the man agement of pain during recovery from minimally invasive cardiac surgery.
Minimally invasive cardiac surgery has significant advantages compared with traditional sternotomy techniques. These include reduced tissue trauma, decreased blood loss, and shorter length of hospital stay.1,2 These procedures are performed through a right-sided anterior minithoracotomy or hemisternotomy. Despite being less invasive than traditional sternotomy, these approaches are associated with significant nociceptive and inflammatory pain,2–4 which is typically managed postoperatively with intravenous opi oid analgesics. Adverse effects of opioids include respiratory de pression, delirium, and gastrointestinal dysfunction, which are particularly problematic in the aging and comorbidpopulation un dergoing cardiac surgery. Analgesic strategies that reduce opioid consumption and improve perioperative outcomes from mini mally invasive cardiac surgery procedures are desirable.
In this case report, we describe successful rescue postoperative analgesiawith the novel application of ultrasound-guided pectoral nerve block types I and II (Pecs I and II) in a patient who underwent mitral valve repair via right anterior minithoracotomy. Pectoral nerve block types I and II, first described by Blanco and colleagues5,6 for patients undergoing breast surgery, are new re gional anesthesia techniques that block the medial and lateral pectoral nerves, second to sixth intercostal nerves, and the long thoracic nerve.7 We report the first description of these blocks for a cardiac surgical patient.
Written consent was obtained from the patient prior to publication of this case report, and the patient approved reporting the case. A 78-year-old man underwent mitral valve repair via rightanterior thoracotomy for severe mitral regurgitation secondary to ruptured chordae tendinae of the posterior leaflet. He had an un eventful intraoperative course and was extubated on postoperative day (POD)1. After extubation, hedescribed10/10 painonthe Numeric Rating Scale (NRS11) at the incision site (Fig. 1) that was minimally responsive to intravenous opioids. He was maintained on a hydromorphone patient-controlled analgesia and scheduled acetaminophen for his analgesia. In addition, he reported difficulty with deep breathing because of pain and required facemask oxygen at 8 L/min to maintain his oxygenation. After obtaining informed consent, we perfomed ultrasound-guided Pecs I and II blocks on the operative side with 30 mL of 0.2% ropivacaine with 1:400,000 epinephrine (Fig. 2). Ten milliliters of this solution was deposited between the pectoralis minor and major muscles at the level of the third rib (Pecs I), and 20 mL was deposited between pectoralis minor and serratus anterior at the level of the fourth rib (Pecs II). Twenty minutes after the block was performed, the patient reported that his pain score had decreased to 2/10 and that he could “breathe easier.” Two hours after the block, his pain score remained at 2/10, and he was weaned completely off supplemental oxygen. For the next 24 hours,his pains core remained at 2/10.He was transitioned from a hydromorphone patient-controlled analgesia to oral oxycodone. On POD 3, he reported worsening incisional pain, and by morning of POD 4, he reported severe 8 to 10/10 incisional pain. He had not received a ketamine infusion or lidocaine infusion adjunctively. He was noted to be splinting and again required supplemental oxygen. After reevaluation of the patient and consent, the Pecs I and II blocks were repeated using 266 mg of liposomal bupivacaine ( Exparel; Pacirea Pharma ceuticals, Inc, Parsippany, New Jersey) diluted in 10 mL of 0.25% bupivacaine (for a total volume of 30 mL) without epinephrine. Ten milliliters was used for Pecs I, and 20 mL was used for Pecs II. Approximately 20 mintues after block placement, the patient reported a pain score of 0/10, and he was again able to oxygenate

FIGURE 1. Depiction of thoractomy incisions in this patient.
well on room air. His pain score remained at 3 to 4/10 over the next 48 hours. He remained at this pain score with oral oxycodone, and the decision was made to not repeat the blocks further. There mainder of his hospital course was extended compared with normal because of a need for an implantable pacemaker for heart block that developed post operatively, and he was discharged home on POD 10.
We report the first successful use of ultrasound-guided Pecs I and II blocks for rescue analgesia in a patient after anterior minithoracotomy for minimally invasive mitral valve surgery. We used the Pecs I andII blocks in the same patient at 2 different time points: first using ropivacaine with epinephrine and subsequently with a mixture of 0.25% bupivacaine and liposomal bupivacaine. This improved the patient’s reported pain scores and allowed for weaning from supplemental oxygen, likely because of improved pulmonary mechanics secondary to the high quality of analgesia.
Minimally invasive cardiac surgery is widely thought to pro duce less postoperative pain and a faster recovery than a traditional sternotomy. However, there are limited data on pain outcomes after these procedures. Several studies of minimally invasive chest sur
gery have demonstrated that postoperative pain can be substantial, and opioid requirements may be high.8 Glower and colleagues 9 reported that postoperative pain was subjectively the same between conventional sternotomy and minimally invasive approaches, al though they note that the pain tended to resolve more quickly in the latter patients. Because pulmonary mechanics in this population are adversely affected by both incisional pain and opioids, quality pain relief relying on nonopioid analgesic strategies is desirable.
Options for effective regional anesthetic techniques are lim ited for patients undergoing minimally invasive cardiac surgery primarily because of the need for systemic heparinization during cardiopulmonary bypass and the risk of postoperative hemodynamic instability. Although neuraxial techniques are well established in non cardiac thoracic surgery to provide superior analgesia compared with systemic opioids10,11 and reduce the incidence of postoperative pulmonarycomplications,12 they are generally avoided in car diac surgery because of the rare but catastrophic risk of epidural hematoma.13 Paravertebral blockade can also be effective for chest wall analgesia, but clinicians may lack the level of expertise or re sources required for its safe performance.14
In contrast, Pecs blockade is a relatively simple fascial plane infiltration technique that seems to be associated with an excellent safety profile.15 These blocks have been described in the setting of breast surgery to provide chest wall analgesia. When combined with general anesthesia for breast surgery, patients who received Pecs blocks had reduced pain scores, reduced opioid consumption for up to 12 hours, and improved sedation scores. Pectoral nerve blocks have also been used in a patient who was a poor candidate for general anesthesia as the primary anesthetic technique for the sub-pectoral implantation of a cardiac resynchronization therapy device.16
Unlike reconstructive breast surgery, which spares injury and manipulation of ribs, pain after anterior thoracotomy is related to
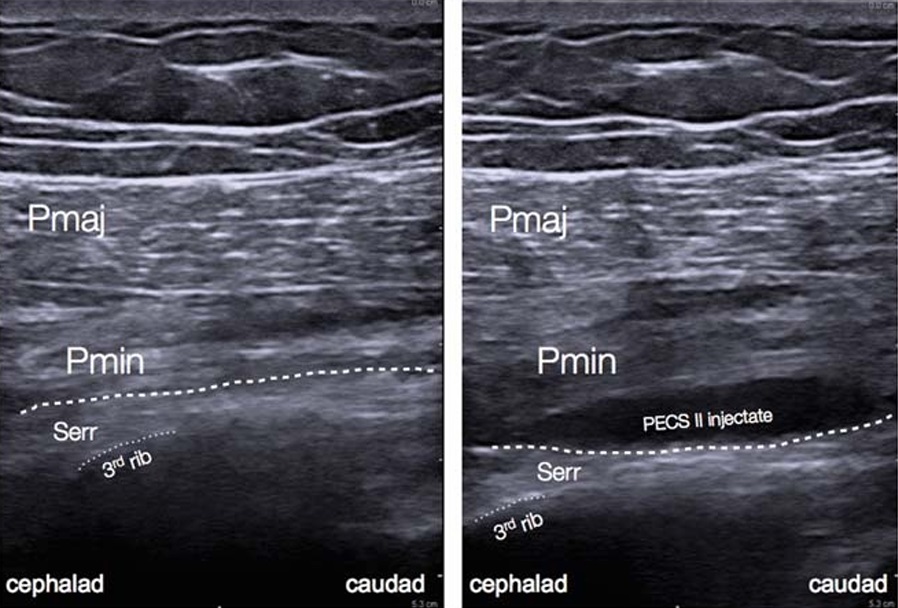
FIGURE2. Preinjection and postinjection sonograms after PecsII block. Note the spread of injectate between the pectoralis minor and serratus anterior muscles. Pmaj indicates pectoralis major muscle;Pmin, pectoralis minor muscle; Serr, serratus anterior muscle. Dashed line indicates fascia between pectoralis minor and serratus anterior muscles. Dotted line traces the third rib.
intraoperative rib retraction, pectoralis and intercostal muscle incisions, and trauma to intercostal nerves. Although there isa paucity of data to support the utility of the Pecs blocks for pain from this mechanism, 1 case report has suggested good anesthesia of the hemithorax after serratus anterior plane block in a patient with multiple rib fractures.17 The Pecs I block targets the medial and lateral pectoral nerves, providing analgesia related to the surgical disruption of the pectoralis major and minor muscles and related fascial structures. The Pecs II block exerts its effect by distributing local anesthetic to the lateral aspect of the chest wall, a mechanism demonstrated in a magnetic resonance contrast study.6 Diffusion through the intercostal muscles provides blockade of the lateral branches of intercostal nerves 2 to 6 and therefore much of the ax illa and anterolateral chest wall and skin. Because Pecs I and II blocks target different structures, we feel it is desirable to perform both techniques for cases such as this. Currently, no prospective studies have been conducted using either the Pecs I and II or serratus anterior blocks in patient populations undergoing procedures in volving rib trauma or manipulation.
The analgesic duration of Pecs blocks is limited by the phar macodynamics of the local anesthetic used. Although our patient hadagoodinitial effect with ropivacaine, the duration of the block was approximately 24 hours. To extend the duration of analgesia,
wechose to repeat the block using liposomal bupivacaine (Exparel) for the subsequent rescue. Liposomal bupivacaine is an extended release formulation that has been shown to provide analgesia for upto 72 hours after a single injection invarious soft-tissue and or thopedic surgical procedures.18 Liposomal bupivacaine may be a particularly useful adjunct in cases where a prolonged duration of action is desired, but a continuous catheter technique is either con traindicated or inconvenient, or where there is a lack of experience and/or infrastructure to place or maintain such catheters. The data oneffectiveness of continuous catheter techniques in fascial plane infiltration blocks are sparse.19 Moreover, placing a catheter im mediately adjacent to the thoracotomy incision would have pre sented a technical challenge and a possible infection risk for our patient. We admixed bupivacaine HCl with the liposomal bupivacaine in order to achieve an immediate effect, a practice that has been shown to be both safe and effective.20,21
In this case report, we demonstrated successful analgesia after minimally invasive cardiac surgery with ultrasound-guided single-shot Pecs I and II blocks, which was further extended using liposomal bupivacaine. There is a lackof datausing Pecs blocks in this patient population, and currently, the role of the blocks might be limited to those patients in whom traditional analgesic tech niques have failed. Although we believe that these blocks may provide an important nonopioid option for the management of pain during recovery from minimally invasive cardiac surgery, a prospective trial is necessary to evaluate the efficacy of these blocks in the cardiac surgery patient population.
1. Soltesz EG, Cohn LH. Minimally invasive valve surgery. Cardiol Rev.
2007;15:109–115.
2. Furrer M, Rechsteiner R, Eigenmann V, Signer C, Althaus U, Ris HB.
Thoracotomy and thoracoscopy: postoperative pulmonary function, pain
and chest wall complaints. Eur J Cardiothorac Surg.1997;12:82–87.
3. Chelly JE, Ghisi D, Fanelli A. Continuous peripheral nerve blocks in acute
pain management. Br J Anaesth. 2010;105(suppl 1):i86–i96.
4. Gammie JS, Zhao Y, Peterson ED, O’Brien SM, Rankin JS, Griffith BP. J.
Maxwell Chamberlain Memorial Paper for Adult Cardiac Surgery.
Less-invasive mitral valve operations: trends and outcomes from the
Society of Thoracic Surgeons Adult Cardiac Surgery Database.
Ann Thorac Surg. 2010;90:1401–1408. 1410.e1; discussion 1408–1410.
5. Blanco R. The ‘Pecs block’: a novel technique for providing analgesia after
breast surgery. Anaesthesia. 2011;66:847–848.
6. Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs
II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol
Reanim. 2012;59:470–475.
7. Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal
analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth
Pain Med.2015;40:68–74.
8. Yamada T, Ochiai R, Takeda J, Shin H, Yozu R. Comparison of early
postoperative quality of life in minimally invasive versus conventional
valve surgery. JAnesth. 2003;17:171–176.
9. Glower DD, LandolfoKP, Clements F, et al. Mitral valve operationvia port
access versus median sternotomy. Eur J Cardiothorac Surg. 1998;
14(suppl 1):S143–S147.
10. Soto RG, Fu ES. Acute pain management for patients undergoing
thoracotomy. Ann Thorac Surg. 2003;75:1349–1357.
11. Ochroch EA, Gottschalk A. Impact of acute pain and its management for
thoracic surgical patients. Thorac Surg Clin. 2005;15:105–121.
12. Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality
and morbidity with epidural or spinal anaesthesia: results from overview of
randomised trials. BMJ. 2000;321:1493.
13. Rosen DA, Hawkinberry DW 2nd, Rosen KR, Gustafson RA, Hogg JP,
Broadman LM. An epidural hematoma in an adolescent patient after
cardiac surgery. Anesth Analg. 2004;98:966–969. table of contents.
14. Ganapathy S,MurkinJM,BoydDW,DobkowskiW,MorganJ.Continuous
percutaneous paravertebral block for minimally invasive cardiac surgery.
J Cardiothorac Vasc Anesth. 1999;13:594–596.
15. Chin KJ, McDonnell JG, Carvalho B, Sharkey A, Pawa A, Gadsden J.
Essentials of our current understanding: abdominalwall blocks. Reg Anesth
Pain Med. 2017;42:133–183.
16. Fujiwara A,KomasawaN,MinamiT.Pectoralnerves(Pecs)andintercostal
nerve block for cardiac resynchronization therapy device implantation.
Springerplus. 2014;3:409.
17. Kunhabdulla NP, Agarwal A, Gaur A, Gautam SK, Gupta R, Agarwal A.
Serratus anterior plane block for multiple rib fractures. Pain Physician.
2014;17:E651–E653.
18. Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine
extended-release liposome injection for prolonged postsurgical analgesia in
patients undergoing hemorrhoidectomy: a multicenter, randomized,
double-blind, placebo-controlled trial. Dis Colon Rectum.2011;54:
1552–1559.
19. Niraj G, Kelkar A, Jeyapalan I, et al. Comparison of analgesic efficacy of
subcostal transversus abdominis plane blocks with epidural analgesia
following upper abdominal surgery. Anaesthesia. 2011;66:465–471.
20. Gadsden J, Long WJ. Time to analgesia onset and pharmacokinetics after
separate and combined administration of liposome bupivacaine and
bupivacaine HCl: considerations for clinicians. Open Orthop J. 2016;10:
94–104.
21. Kharitonov V. A review of the compatibility of liposome bupivacaine with
other drug products and commonly used implant materials. Postgrad Med.
2014;126:129–138.
