The use of the term standard of care is common and established in law. Although varying by state, the definition typically in cludes “the caution that a reasonable person in similar circum stances would exercise in providing care to a patient.”1,2 Establishing that a physician has deviated from the standard of care is the central objective of the plaintiff’s attorney for the suc cessful litigation of a medical malpractice case.1,2 The current standard of care in the United States is to perform regional anes thesia in patients who are awake or are under conscious sedation rather than general anesthesia (GA).3 Presumably, this practice has evolved from the notion that regional anesthesia is safer when patients can communicate signsand symptomsof higher-risk needle and local anesthetic locations.3 However, with improvements in technology and increased provider expertise, we speculate that our current practice today relies far less on patients’ feedback and more on objective measures. Additionally, even when a paresthesia is elicited during needle advancement or injection in a patient who is awake, research has shown that this is not predictive of peripheral nerve injury.3 Furthermore, there are particular patient populations for which the benefits of ensuring cooperation and immobility outweigh the risk of performing regional blocks under GA.3 By sharing our interdisciplinary practice changes around pectoralis blocks, we dare to suggest that certain subsets of patients would benefit from the liberalization of this standard toward performing more regional anesthesia under GA. Revisiting this age-old debate initially began after our institution altered its policy of performing all regional anesthesia in awake or in consciously sedated patients to accommodate the per formance of pectoralis blocks after the induction of GA. This change was implemented after our quality assurance follow-up process revealed that upholding this standard of care was, in fact, compromising the patient care experience. Before this change, our practice was to offer preoperative pectoralis I and II blocks (Pec) under conscioussedation for patients undergoing mastectomy sur gery.4–7 Surgery for breast cancer is understandably a stressful and emotional time for both patients and families. In this setting, we experienced 2 distinct phenomena. First, many of our patients were interested in a regional nerve block for pain control, but the thought of receiving multiple injections in to the breast(s)under conscious sedation was deemed unacceptable. Second, compared with our other regional techniques, we found a disproportionate number of patients experiencing discomfort and dissatisfaction centered on the technical performance of the Pec block. Despite what seemed to be generous use of midazolam, fentanyl,and local anesthetic, our quality assurance follow-up process revealed multiple patients who would not repeat the block if additional surgery was needed.
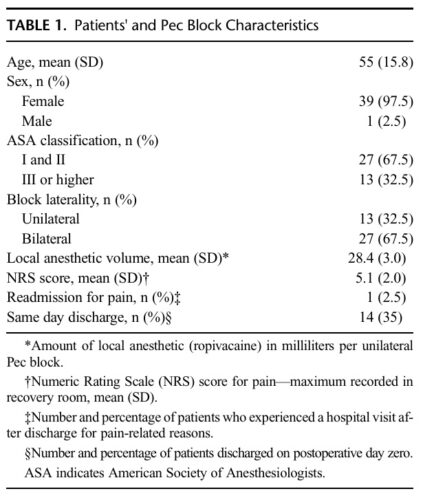
Based on an interdisciplinary improvement project conducted with our nursing and surgical colleagues, we postulated that our patients would be better served if we performed Pec blocks after the induction of GA in the operating room. Our cadaveric dissections suggested that a Pec block istechnically a fascial plane block similar in anatomical features to that of a transversalis plane block, which is the only block we have been performing routinely under GA.8,9 We created a defined care pathway and scheduled structured operating room time that allowed us to provide the Pec blocks under GA. As a contributing member of the International Registry of Regional Anesthesiology, our data collection and follow-up process is institutional review board–approved and has been described in previous publications.10–12 Between July 1, 2015, and January 31, 2016, we performed 40 Pec blocks under GA. Table 1 summarizes patients’ demographics and block characteristics of this group. Twenty-seven patients underwent bi
lateral breast procedures and received bilateral Pec blocks. Per the International Registry of Regional Anesthesiology follow-up process, we did not identify any quality-compromising issues immediately, during the postanesthesia care recovery or during the postdischarge follow-up.10–12 Although our small case series was not powered to show a difference in safety or quality in this “before” and “after” time period, we found that our practice change provided a natural segue into reopening the discussion about per forming peripheral nerve blocks in patients who are asleep.
Weneed to return to the start of the century to find the basis for the asleep versus awake debate. In 2000, Benumof13 authored a case series describing 4 patients who developed permanent loss of cervical cord function after what were thought to be interscalene nerve blocks performed using anatomic landmark technique under GAorheavysedation.Thesecaseswere, infact, intraneuronal injections of local anesthetic that caused permanent neurologic dysfunction. Benumofpostulated that these devastating complications could have been prevented if those patients were awake enough to voice concern about a disproportionate level pain with injection; and, therefore, concluded that GAwas a relative contra indication for interscalene blocks. Ironically, one of the main con clusions he rendered from this case series was that physicians “should ensure [that] patient[s]… [will]… not unexpectedly move.”13
These cases followed the 1998 report of a patient who experi enced partial paralysis after an epidural placed under GA.14 The authors concluded:
This case reinforces the admonition against attempting epidural puncture above the termination of the cord in unconscious, areflexic patients, and the opinion that the risk of such gravity is only justified as a life-saving measure under exceptional circumstances.14
The results in the aforementioned reports were truly cata strophic and changed the regional anesthesia practice of many physicians. This topic has been debated over the years and was re cently addressed by the Second ASRAPractice Advisoryon Neu rological Complications.3 In the executive summary, the practice advisory acknowledged the controversy about blocks in heavily sedated patients or in patients under GA, and went on to cite a report from the American Society of Anesthesiologists Closed Claims study that showedanincrease ininjury rate inanesthetized or heavily sedated patients who had cervical interventional pain procedures.3 The 2015 executive summary continued to support their initial recommendation to avoid performing regional anes thesia or interventional pain medicine procedures in anesthetized or deeply sedated patients.3 However, they alsomentionedthat exceptions to this recommendation may be made if, in certain adult populations, the benefit of ensuring cooperation and immobility outweighed the risk of performing these procedures under deep sedation or GA (much like in the case of pediatric patients).3 This recommendation referenced developmentally delayed patients and those with multiple bone traumas as suitable exceptions, but could easily be extended to include demented patients, those with severe anxiety or chronic pain, and those with an already compro misedsensorium (patients whowould likely not be abletoprovide appropriate/adequate feedback about paresthesias even if awake). What the advisory failed to comment on was the continued routine placement of spinal cord stimulators in patients with chronic pain or epidurals for ventilator weaning in patients with chest trauma under GA—both procedures for which other sedation op
tions provide inferior working conditions.15,16
Given the fortunate rarity of procedural related neurological injury, it is unlikely that we will ever have randomized controlled data comparing regional anesthesia performed in patients who are awakeversus those under GA. However, there is reassuring observational data from the Pediatric Regional Anesthesia Network that demonstrated no increases in postoperative neurologic symptoms in pediatric patients who had blocks placed under GA when compared to awake or sedated patients.17 A total of 53,564 blocks were analyzed, and approximately one third of these blocks were placed in children between 10 and 18 years of age.17 A second paper published by the same group focused on interscalene blocks, historically the most controversial, and found that the rate of postoperative neurologic symptoms was nearly identical between GA and conscious sedation groups.18 One wonders if such findings would also translate to adults where there actually may be more anatomical room for error (ie, increased space between the brachial plexus and neuraxis given the larger adult size).
Although the idea of performing nerve blocks under GA is acceptable for pediatric patients, this is partly due to the unsafe nature of doing so in the awake child given their inability to remain cooperative and immobile. Wakefulness in the adult population is used as a monitor for safety because they are typically able to effectively communicate if/when a paresthesia is elicited. However, studies have shown that not all paresthesias are predictive of pe ripheral nerve injury and that not all nerve injury is proceeded by paresthesia.3 With this in mind, using more objective measures of safety, independent of patient participation and level of provider skill, may be more appropriate in preventing nerve injury. More recently, opening injection pressure has been studied as a measure of needle and nerve contact. In 2014, Gadsden et al19 found that the injection of solution 1 mm from a brachial plexus root during an interscalene nerve block resulted in lowopening injection pres sure (8.2 psi), whereas injection with the needle against the root resulted in high opening injection pressure (20 psi). This finding waslater confirmed by a second study involving the femoral nerve where Gadsden verified that opening injection pressures greater than 15 psi were associated with a needle tip position slightly in denting the epineurium of the femoral nerve, whereas opening injection pressures less than 15 psi were associated with needle tip
positions not indenting these structures.20 Although not ideal as a sole monitor, combining several objective measures for safety may be an adequate substitute for wakefulness in certain subsets of patients.
With recent advancements in ultrasound technology (eg, 3-dimensional, compounding, automated needle guidance, pure wave crystals), our ability to detect high-risk needle locations and to im age non-neural landmarks is improving. Superior image resolution has given us the ability to distinguish intimate neural tissue layers from surrounding connective tissue. However, despite the implementation of ultrasound and these improvements in technology, the incidence of peripheral nerve injury has remained the same.21 Although we continue to improve on needle placement strategies that may minimize the risk of unintended intrafascicular injection, we speculate that visualization of neural tissue can lead to a false sense of security with regard to needle advancement in areas that are in close proximity to desired targets. However, we do believe that recent regional anesthetic practice has evolved to the point where safe and effective nerve blocks are often a function of local anesthetic deposition in targeted fascial planes rather than into named neural structures. In recent years, many practitioners have moved toward injecting local anesthetics at significant distances from traditionally described neural structures. In 2011, Spence et al22 suggested that for interscalene nerve blocks, injecting local anesthetic more conservatively into a periplexus lo cation resulted in an equally effective sensory and motor blockade when compared to a more traditional and aggressive intra-plexus injection. Findings from another study authored by Brull et al23 in 2014 further supported this less aggressive approach, suggesting that minimizing needle-to-nerve root distance was not necessary to achieve effective analgesia when using ultrasound guidance for interscalene blocks.
Although a definitive answer regarding the safety portfolio of performing asleep regional anesthesia in adults eludes us, there exist significant data to suggest that we should at least re-engage in a healthy debate regarding who, what, where, and why. We leave this as an open-ended question to generate conversation among our colleagues. We hope the readership appreciates that there needs to be room for introducing flexibility into our current stan dard of practice to allow for exceptions in particular subsets of patients when it may be appropriate.
1. Moffett P, Moore G. The standard of care: legal history and definitions: the
bad and good news. West J Emerg Med. 2011;12:109–112.
2. Bal BS. An introduction to medical malpractice in the United States.
Clin Orthop Relat Res. 2009;467:339–347.
3. Neal JM, Barrington MJ, Brull R, et al. The second ASRA practice
advisory on neurologic complications associated with regional anesthesia
and pain medicine: executive summary 2015. RegAnesthPainMed.2015;
40:401–430.
4. Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block:
a novelultrasound-guided thoracic wall nerve block. Anaesthesia.2013;68:
1107–1113.
5. Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal
analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth
Pain Med. 2015;40:68–74.
6. Pérez MF, Duany O, de la Torre PA. Redefining PECS blocks for
postmastectomy analgesia. Reg Anesth Pain Med. 2015;40:729–730.
7. Turbitt L,NelliganK,McCartneyC.Pectoralnerve blocks for breastcancer
surgery: a methodological evaluation. Reg Anesth Pain Med.2015;40:
388–389.
8. Young MJ, Gorlin AW, Modest VE, Quraishi SA. Clinical implications of
the transversus abdominis plane block in adults. Anesthesiol Res Pract.
2012;2012:731645.
9. Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus
abdominis plane (TAP) blocks for analgesia after abdominal surgery.
Cochrane Database of Syst Rev. 2010: CD007705.ß.
10. Barrington MJ. International Registry of Regional Anaesthesia; 2015.
Available at: http://www.anaesthesiaregistry.org.
11. Barrington M, Watts SA, Gledhill SR, et al. Preliminary results of the
Australasian Regional Anaesthesia Collaboration: a prospective audit of
morethan7000peripheralnerveandplexusblocksforneurologicandother
complications. Reg Anesth Pain Med. 2009;34:534–541.
12. Sites BD, Taenzer AH, Herrick MD, et al. Incidence of local anesthetic
systemic toxicity and postoperative neurologic symptoms associated with
12,668 ultrasound-guided nerve blocks: an analysis from a prospective
clinical registry. Reg Anesth Pain Med. 2012;37:478–482.
13. Benumof JL. Permanent loss of cervical spinal cord function associated
with interscalene block performed under general anesthesia.
Anesthesiology. 2000;93:1541–1544.
14. Bromage PR, Benumof JL. Paraplegia following intracord injection during
attempted epidural anesthesia under general anesthesia. Reg Anesth Pain
Med. 1998;23:104–107.
15. Falowski SM, Celii A, Sestokas AK, Schwartz DM, Matsumoto C, Sharan
A.Awakevs. asleep placement of spinal cord stimulators: a cohort analysis
of complications associated with placement. Neuromodulation. 2011;14:
130–135.
16. Gadsden J, Warlick A. Regional anesthesia for the trauma patient:
improving patient outcomes. Local Reg Anesth.2015;8:45–55.
17. Taenzer AH, Walker BJ, Bosenberg AT, et al. Asleep versus awake: does it
matter? Pediatric regional block complications by patient state: a report
from the Pediatric Regional Anesthesia Network. RegAnesthPainMed.
2014;39:279–283.
18. Taenzer A, Walker BJ, Bosenberg AT, et al. Interscalene brachial plexus
blocks under general anesthesia in children: is this safe practice? A report
from the Pediatric Regional Anesthesia Network (PRAN). Reg AnesthPain
Med. 2014;39:502–505.
19. Gadsden JC, Choi JJ, Lin E, Robinson A. Opening injection pressure
consistently detects needle-nerve contact during ultrasound-guided
interscalene brachial plexus block. Anesthesiology. 2014;120:1246–1253.
20. Gadsden JC, Malikah L, Levine DM, Robinson A. High opening injection
pressure is associated with needle-nerve and needle-fascia contact during
femoral nerve block. Reg Anesth Pain Med.2016;41:50–55.
21. Neal JM. Ultrasound-guided regional anesthesia and patient safety: an
evidence-based analysis. Reg Anesth Pain Med. 2010;35:S59–S67.
22. Spence BC, Beach ML, Gallagher JD, Sites BD. Ultrasound‐guided
interscalene blocks: understanding where to inject the local anaesthetic.
Anaesthesia. 2011;66:509–514.
23. Brull R, Kirkham KR, Taffé P, et al. The maximum effective
needle-to-nerve distance for ultrasound-guided interscalene block:
an exploratory study. Reg Anesth Pain Med. 2014;39:56–60.
