Background and Objectives: The purpose of this prospective open label study was to investigate the analgesic effects of an ultrasound guided greater occipital nerve (GON) block at the level of C2, as the nerve courses superficially to the obliquus capitis inferior muscle.
Methods: Patients with a diagnosis of occipital neuralgia or cervicogenic headache were recruited for the study. Ultrasound-guided GON blocks at the level of C2 were performed by experienced clinicians according to a standardized protocol. Numeric rating scale pain scores were recorded preinjection and at 30 minutes, 2 weeks, and 4 weeks after injection.
Results: A total of 14 injections were performed with a mean procedure time of 3.75 minutes. Anesthesia in the GON distribution was achieved for 86% of patients at 30 minutes postinjection. Compared with baseline, numeric rating scale scores decreased by a mean of 3.78 at 30 minutes
(P < 0.001), 2.64 at 2 weeks (P = 0.006), and 2.21 at 4 weeks (P = 0.01). There were no significant adverse events reported during the study period.
Conclusions: This prospective open-label study demonstrated successful blockade of the GONat the level of C2 using a novel ultrasound-guided tech nique. Significant reductions in pain scores were observed over the 4-week study period, and no adverse events were reported. The observations from this study provide important preliminary data for future randomized trials in volving patients with occipital neuralgia and cervicogenic headache.
(Reg Anesth Pain Med 2017;42: 99–104)
Participants and Setting
The study was approved by the Mayo Foundation Institu tional Review Board, and written informed consent was obtained from all study participants. All potential subjects were referred to the Mayo Pain Clinic in Rochester, Minnesota, for a therapeutic
GONinjection. Inclusion criteria included (1) unilateral headache symptoms attributed to occipital neuralgia or cervicogenic head acheasdefinedbyIHS’sInternational Classification of Headache Disorders (second edition)15 and (2) age 18 years or older (no up per age limit defined). Exclusion criteria included (1) bilateral oc cipital neuralgia or cervicogenic headache symptoms; (2) history of cervical spine surgery, trauma, or surgical procedure involving the head or neck during the last year; (3) use of new analgesic medications within 24 hours prior to study enrollment; (4) evi dence of impaired sensation in the GON dermatome region (pos terior scalp to the vertex of the cranium) from neurological, dermatological, or other disease process; (5) evidence of cranial defect or other anatomical abnormality near the target injection site; (6) history of bleeding diathesis, coagulopathy, or current use of anticoagulant medications; (7) pregnancy; (8) history of ad verse reaction or allergy to local anesthetic agents or corticoste roids; and (9) occipital nerve block within the past 3 months.
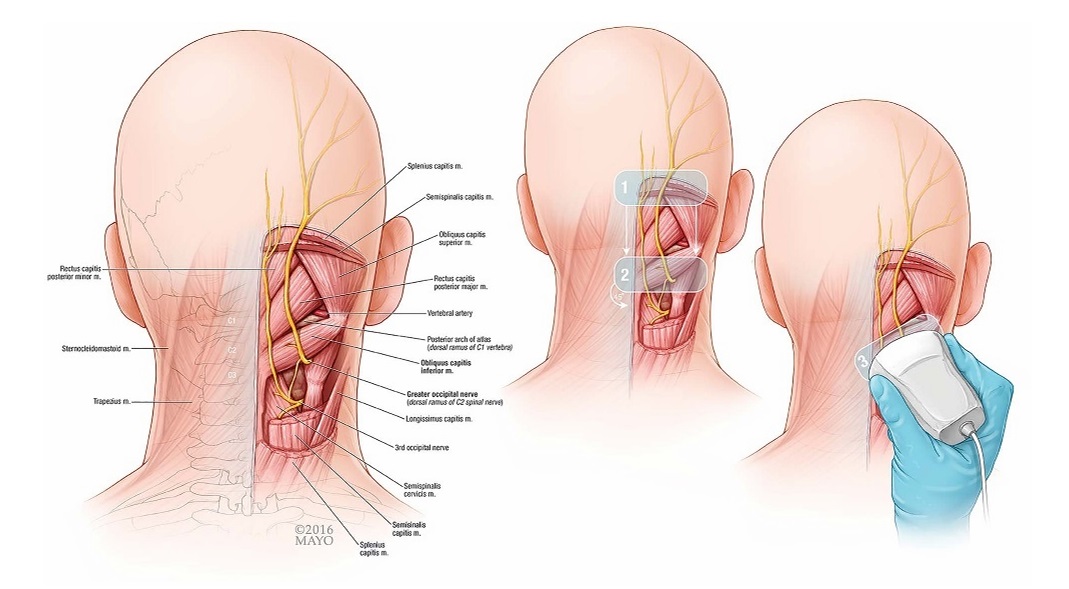
FIGURE1. Theillustrationontheleftdepictsrelevantcervicalanatomy.The2imagesontherightoutlinepr perultrasoundprobepositioning for a GONblock at the level of C2. 1, Axial over EOP; 2, axial over C2 spinous process; 3, oblique in-plane with OCI muscle.
Injection Technique
Subjects were placed in the prone position with head and neck flexed to compensate for normal lordosis of the cervical spine. The external occipital protuberance (EOP) was identified and palpated on each patient. The ultrasound transducer (Phillips CX-50 ultrasound machine, 6–12 MHz high-resolution linear transducer; Philips North America, Andover, Massachusetts) was initially placed in the transverse orientation at midline to identify the EOP (Figs. 1 and 2). The transducer was moved caudally over the location of C1 to locate the C2 spinous process as identified by its bifid appearance. Once C2 was properly identified, the transducer was moved laterally with the lateral edge of the trans ducer aimed at the transverse process of C1 to identify the OCI muscle. The GONwasidentified lying superficial to the OCI, traversing the muscle caudal to rostral and lateral to medial. Prior to
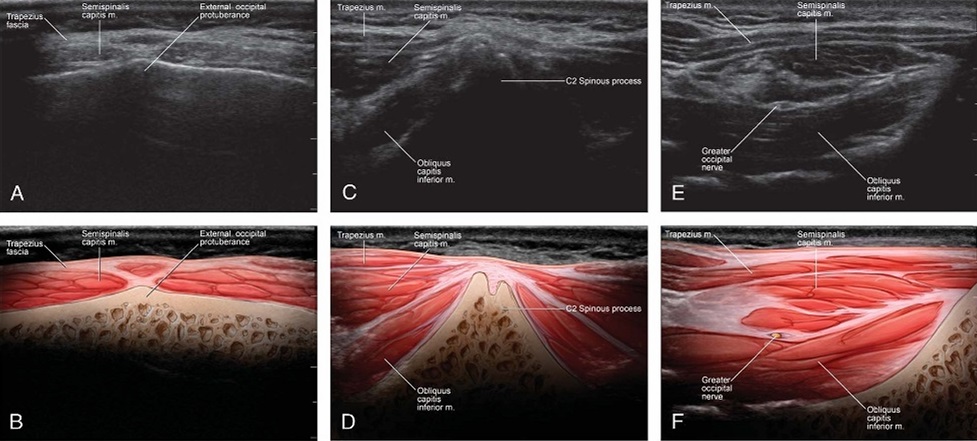
FIGURE2.Ultrasound images and corresponding illustrations depicting relevant anatomy over the EOP(A,B), bifid C2 spinous process(C,D), and OCI muscle (E, F).
needle placement, the presence or absence of vascular structures as measured with Doppler ultrasound was recorded. The needle was advanced in-plane with the transducer from medial to lateral underdirect ultrasound visualization until the tip was visualized in the fascial plane between the OCI and SC (Fig. 3). A 25-gauge, 2-inch spinal needle was used for all injections. A total of 4 mL consisting of 1 mL of 2% lidocaine, 2.5 mL of 0.25% bupivacaine, and 3 mgof betamethasonewas injected. The spread of injectate was visualized as it encompassed the GON between the 2 muscles.
Assessments
Demographic and Clinical Characteristics
Demographicinformation was collected from each patient, in cluding age, race, sex, height, weight, and body mass index (BMI). Information was also collected regarding the laterality of symp toms, duration of symptoms, and use of analgesic medications.
Determination of Successful GON Block
Thirty minutes following the injection, each patient was examined by a physician not directly involved in the study protocol. A successful block was defined as the absence of light-touch sensation in the dermatome of the GON.
Pain Intensity
Pain intensity was assessed preinjection, 30 minutes postin jection, 2 weeks postinjection, and 4 weeks postinjection using a numeric rating scale (NRS) marked from 0 to 10 with fixed inter vals.16 The preinjection and 30-minute postinjection pain ratings were obtainedinthe pain clinic. The 2- and4-week follow-up pain ratings were obtained by telephone.
Adverse Events
At all follow-up time points after the GON block, we inquired about the occurrence of adverse events via telephone. Symptoms
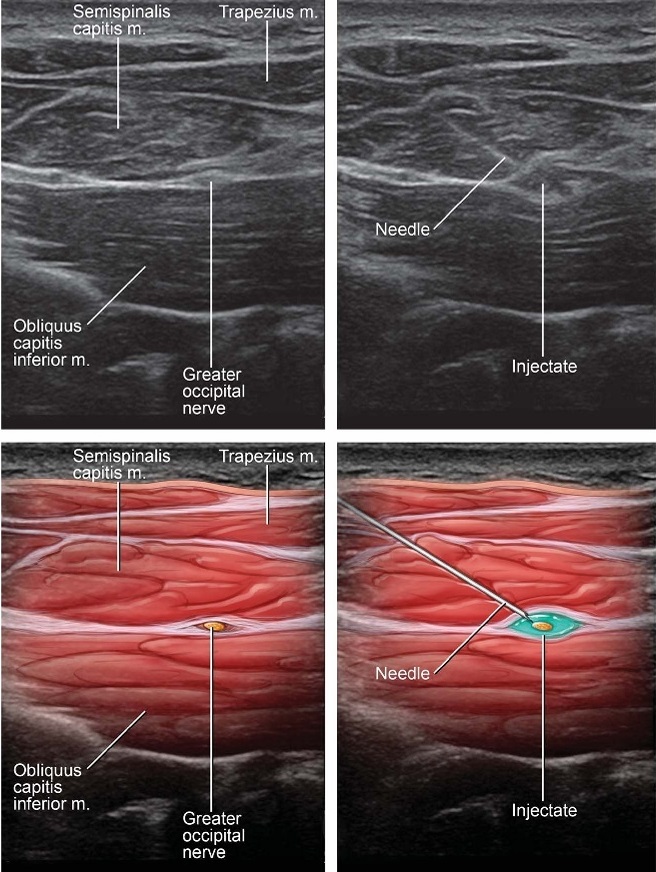
FIGURE 3. Needle visualization utilizing an in-plane medial to lateral approach to target the GON as it courses over the OCI muscle.
addressed included dizziness, blurred vision, slurred speech, lightheadedness, paresthesias, seizure activity, and headache.
Statistical Plan
Sample Size Calculation
Based on prior studies validating numerical pain scales, we considered a clinically significant improvement to be at least 2.0 units on a 10-point scale.17,18 We calculated that approximately 15 patients would be needed to detect a mean change of 2.0 units with α =0.05andβ = 0.80, assuming an SD of 2.0 units at each time point.
Data Analysis
Data analysis for this study was largely descriptive, including percentages and counts for categorical data and medians with 25th to 75th interquartile ranges (IQRs) for continuous data. The primary outcome measure was the change in NRS score from base line to week 4. The secondary outcomes were the change in NRS score from baseline to 30 minutes postprocedure, change in NRS score from baseline to week 2, and the occurrence of ad verse events. The change in NRS scores was assessed using
Wilcoxon signed rank tests with the level of significance set at P <0.05. Data were analyzed using Microsoft Excel (Microsoft, Redmond, Washington).
Demographic and Clinical Characteristics
The demographic and clinical characteristics are summarized in Table 1. Fifteen patients were initially recruited to participate in the study, one of whom was excluded at the time of preinjection consult because of a cervical fusion present at C2. Of the 14 re maining patients, 50% were male (n = 7), and all were white.
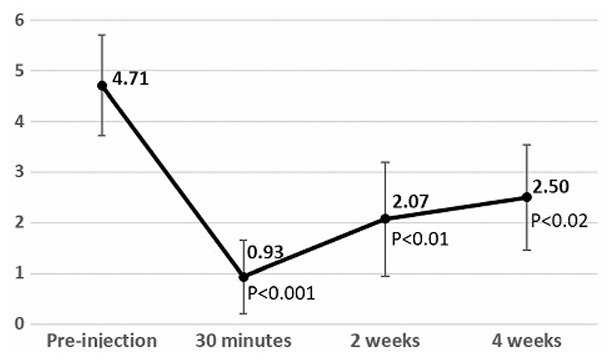
FIGURE 4. MeanNRSpain scores following injection. Measurements taken preinjection, 30 minutes postinjection, 2 weeks postinjection, and 4 weeks postinjection. Bars represent 95%confidenceintervals. P values listed for Wilcoxon signed rank tests compared with preinjection scores.
The mean age was 61.1 (SD, 17.4) years, and the majority of patients had a diagnosis of occipital neuralgia. Thirty-six percent of patients (n = 5) were taking medications for headache prophylaxis at the time of injection. This included 1 patient using carbamaze pine, 1 using topiramate, 1 using gabapentin, and 1 patient using both verapamil and valproicacid. The fifthpatient was onprophy lactic Botox injections every 3 months. Ninety-three percent of patients (n = 13) were taking short-acting pain medications as needed for symptom control, including acetaminophen (n = 8), nonsteroidal anti-inflammatory drugs (n = 6), triptans (n = 3), combination antimigraine formulations (n = 1), and opioids (n = 1).
Block Success
Each procedure took an average of 3.75 (SD, 1.67) minutes to complete after ultrasound visualization of the nerve. A success ful block was obtained in 86% of patients (n = 12) as defined by the absence of light-touch sensation in the dermatomal distribu tion of the GON 30 minutes following the injection.
Pain Intensity
MeanNRSpainratingatbaselinewas4.71, with amedianof 5 and IQR of 3 to 6 (Fig. 4). Compared with baseline, the mean NRS score at week 4 was 2.50 (P < 0.02), with a median of 2 and IQRof1to3.75.MeanNRSscoresat 30 minute sand 2 weeks post procedure were 0.93 (median, 0.50; IQR, 0–1; P <0.001)and 2.07 (median, 2; IQR, 0–3.50; P < 0.01), respectively.
Adverse Events
No adverse events related to the injection were reported, in cluding dizziness, blurred vision, slurred speech, lightheadedness, paresthesias, pseudoseizure, hypertension, or headache.
We demonstrate the safety and efficacy of an ultrasound guided GON block using a proximal approach to target the nerve at the level of C2 as it courses between the SC and OCI muscles. In this study, the GON was successfully blocked at the time of in
jection with significant differences in pain scores after 4 weeks. Prior clinical studies demonstrated efficacy of ultrasound-guided GON blocks via the standard approach at the superior nuchal line.12–14 This is the first study tovalidate the technique described
by Greher et al5 in a clinical setting with patients referred for oc cipital neuralgia or cervicogenic headache. There were no adverse events reported in our study, although a larger cohort will be needed to confirm a complication rate lower than the 5% to 10% reported for nonguided blocks.10,11
There were several strengths of this study. The procedures were performed by clinicians (M.J.P., S.M.M.) who areveryexpe rienced with ultrasound guidance under an established protocol that ensured standardization. Strict inclusion and exclusion
criteria limited our patient population to those with pain likely at tributed to GON pathology. Interestingly, the clinical criteria for occipital neuralgia and cervicogenic headache have changed since our study began, with the publication of the third edition of the IHS’s The International Classification of Headache Disorders, but the updated criteria would not have excluded any of the patients who participated in our study.6
There is a growing body of evidence showing improved patient outcomes with ultrasound-guided approaches for interven tional pain procedures.19,20 This has prompted research seeking to improve injection techniques by visualizing target structures at more accessible or potentially safer locations. In this case, the GON iseasilyvisualizedat the level of C2asit courses superficial to the OCI musclewith nobony artifacts to distort its sonographic appearance. As the nerve courses distally, it divides into several smaller branches and pierces the trapezius at variable locations,
which may lead to results that are less reproducible.21 In addition, many proposed sites of GON compression by vascular, muscular, or osteogenic causes are at the level of the cervical spine or upper cervical nerve roots.2,4,22,23 The results of this study suggest that anesthetizing the nerve at a more proximal location, closer to these potential areas of compression, may result in improved analgesic effect.
The goal of this study was to demonstrate feasibility and prompt future research comparing this approach to other standard techniques. Limited conclusions can be drawn from a small study without blinding to intervention or a control group. However, the sample was large enough to demonstrate a significant decrease in pain, a high success rate of GON blockade, and a lack of signifi cant adverse events. The patient population had an average age older than 60 years and was entirely white, which may limit the reproducibility of our results in other settings. However, our sub jects had an average BMI similar to the figures reported for American adults.24 Although only 2 cases of cervicogenic head ache were included in the study, patients had varying clinical pre sentations in terms of duration of symptoms, location of pain, and medications used.
Other limitations of this study include a follow-up of only 4 weeks following injection, although this is comparable to similar studies.10,12 We know of 1 patient whose pain recurred after the study period, suggesting that the improvement in pain
scores seen after 4 weeks may not be maintained long term. However, this outcome is not unexpected, given our experience with other corticosteroid injections in which the analgesic ef fect wanes over time. We anticipate that injections will need
to be repeated periodically. In addition, our primary outcome measure of the NRS pain score is subjective and potentially af fected by many factors, including comorbid pain conditions, timing of analgesic medication, and psychosocial factors.
Another relevant consideration is the possibility of local anes thetic spread to important structures in close proximity to the OCI. These structures include both the C1 and C3 primary dorsal rami and the origin of the third occipital nerve. The incidence of inadver
tent local anesthetic spread is unknown, but the use of ultrasound guidance and small volumes of injectate likely mitigate this risk. In contrast, traditional GON blocks that utilize anatomic landmarks with higher injectate volumes are likely to anesthetize adjacent structures, including the lesser occipital and great auricular nerves.3
To further quantify the efficacy of this technique, future re search will include a randomized controlled trial comparing it with traditional nonguided injections at the superior nuchal line. In order to address the limitations noted previously, we will in
clude a larger population with a longer follow-up period and a functional outcome scale to supplement the NRS pain score. Con sideration will be given to broadening the patient population to in clude those with bilateral symptoms or with recent procedures involving the head or neck, which were the primary reasons for exclusion from this study. The use of abortive or breakthrough medications will be assessed as a secondary aim to further under stand the impact of these interventions.
ACKNOWLEDGMENTS
The authors thank Dr Michael Hooten for his assistance with
preparation of the manuscript and Mr David Cheney for creating
the illustrations.
1. Tubbs RS, Watanabe K, Loukas M, Cohen-Gadol AA. The intramuscular
course of the greater occipital nerve: novel findings with potential
implications for operative interventions and occipital neuralgia. Surg
Neurol Int. 2014;5:155.
2. Cesmebasi A, Muhleman MA, Hulsberg P, et al. Occipital neuralgia:
anatomic considerations. Clin Anat. 2015;28:101–108.
3. Natsis K, Baraliakos X, Appell HJ, Tsikaras P, Gigis I, Koebke J.
The course of the greater occipital nerve in the suboccipital region: a
proposal for setting landmarks for local anesthesia in patients with
occipital neuralgia. Clin Anat. 2006;19:332–336.
4. Gille O, Lavignolle B, Vital JM. Surgical treatment of greater occipital
neuralgia by neurolysis of the greater occipital nerve and sectioning of
the inferior oblique muscle. Spine (Phila Pa 1976).2004;29:828–832.
5. Greher M, Moriggl B, Curatolo M, Kirchmair L, Eichenberger U.
Sonographic visualization and ultrasound-guided blockade of the greater
occipital nerve: a comparison of two selective techniques confirmed by
anatomical dissection. Br J Anaesth. 2010;104:637–642.
6. The International Classification of Headache Disorders. Third edition
(beta version). Cephalalgia. 2013;33:629–808.
7. Lauretti GR, Corrêa SW, Mattos AL. Efficacy of the greater occipital
nerve block for cervicogenic headache: comparing classical and
subcompartmental techniques. Pain Pract. 2015;15:654–661.
8. Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tawfik OM. Occipital
nerve blockade for cervicogenic headache: a double-blind randomized
controlled clinical trial. Pain Pract.2006;6:89–95.
9. Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tawfik OM. Repetitive
occipital nerve blockade for cervicogenic headache: expanded case report
of 47 adults. Pain Pract. 2006;6:278–284.
10. Weibelt S, Andress-Rothrock D, King W, Rothrock J. Suboccipital nerve
blocks for suppression of chronic migraine: safety, efficacy, and predictors
of outcome. Headache. 2010;50:1041–1044.
11. Sahai-Srivastava S, Subhani D. Adverse effect profile of lidocaine
injections for occipital nerve block in occipital neuralgia. J Headache Pain.
2010;11:519–523.
12. Palamar D, Uluduz D, Saip S, Erden G, Unalan H, Akarirmak U.
Ultrasound-guided greater occipital nerve block: an efficient technique in
chronic refractory migraine without aura? Pain Physician. 2015;18:
153–162.
13. ShimJH,KoSY,BangMR,etal.Ultrasound-guidedgreateroccipitalnerve
block for patients with occipital headache and short term follow up.
Korean J Anesthesiol.2011;61:50–54.
14. Vanderhoek MD, Hoang HT, Goff B. Ultrasound-guided greater occipital
nerve blocks and pulsed radiofrequency ablation for diagnosis and
treatment of occipital neuralgia. Anesth Pain Med. 2013;3:256–259.
15. Headache Classification Subcommittee of the International Headache
Society. The International Classification of Headache Disorders. Second
edition. Cephalalgia. 2004;24(suppl 1):9–160.
16. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating
scale as a screening test in primary care. J Gen Intern Med.2007;22:
1453–1458.
17. Bird SB, Dickson EW. Clinically significant changes in pain along the
visual analog scale. Ann Emerg Med. 2001;38:639–643.
18. Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically
important changes in pain severity measured on a visual analog scale.
Ann Emerg Med. 2001;38:633–638.
19. Neal JM. Ultrasound-guided regional anesthesia and patient safety: an
evidence-based analysis. RegAnesthPainMed.2010;35:S59–S67.
20. Neal JM, Brull R, Chan VW, et al. The ASRA evidence-based medicine
assessment of ultrasound-guided regional anesthesia and pain medicine:
executive summary. RegAnesthPainMed. 2010;35(suppl 2):S1–S9.
21. Loukas M, El-Sedfy A, Tubbs RS, et al. Identification of greater occipital
nerve landmarks for the treatment of occipital neuralgia. Folia Morphol
(Warsz). 2006;65:337–342.
22. Manolitsis N, Elahi F. Pulsed radiofrequency for occipital neuralgia. Pain
Physician.2014;17:E709–E717.
23. Vanelderen P, Lataster A, Levy R, Mekhail N, van Kleef M, van Zundert J.
8. Occipital neuralgia. Pain Pract. 2010;10:137–144.
24. Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children
and adults: United States, 2007–2010. VitalHealthStat. 2012:1–48.
