Abstract: Despite its popularity, ultrasound (US)–guided regional anes the siology is associated with significant limitations. The latter can be attrib uted to either the US machine (ie, decreased ability to insonate deep neural structures, as well as the thoracic spine) or the operator. Shortcomings as sociated with the operator can be explained by errors in perception (ie, am biguous criteria for needle/catheter tip–to–nerve proximity and subparaneural local anesthetic injection) or interpretation. Perhaps the greatest confusion afflicting US-guided regional anesthesiology originates from an intellectual misconception pertaining to its application. Increasingly, authors are using US to identify interfascial planes where local anesthetic can be injected thereby “discovering” new truncal blocks. Often these novel blocks suffer from a lack of proper randomized, comparative validation.
Fortunately, solutions have been proposed to remedy many short comings associated with US guidance. The inability of US to reliably insonate deep neural structures can be circumvented with adjunctive neurostimulation. Fluoroscopy and waveform analysis have been proven to increase the success rate of thoracic epidural blocks. For con tinuous nerve blocks, combined US-neurostimulation may provide an objective end point (ie, an evoked motor response) for neural proximity and subparaneural positioning of the catheter tip. Finally, the solution to the plethora of nonvalidated US-guided blocks is both elegant and simple. New nerveblocksshouldanswer aspecific clinical need, and their first descriptions should take the form of an adequately powered, observer blinded, randomized comparison against the established standard of care or, at the very least, a large case series (eg, a Brief Technical Report).
(Reg Anesth Pain Med 2017;42: 556–563).
The first randomized trial to formally investigate ultrasonography (US) for peripheral nerve blocks can be traced back to 1994.1
However, US guidance only found its place in the anesthesiologist’s armamentarium after the dawn of the new millennium.2 Despite the slow start, the clinical pendulum has indisputably shifted toward the camp of US,3which has emerged as the dominant adjunct for nerve blocks.4
In fact, US currently plays such a central role within the specialty that US-guided regional anesthesiology now benefits from its own, unique acronym(UGRA).5 This hase venpromptedsome experts to advocate abandoning traditional nerve localization mo dalities altogether.6,7 Furthermore, the recent introduction of point-of-care ultrasonography (POCUS) into operating room settings suggests that adoption of US technology willgain even more momentum in years to come.8
As clinicians, teachers, researchers, and editorial board members, we believe that the time has come to ask ourselves (aswell as the community of regional anesthesiologists) the following ques tions: (1) Has the pendulum swung too far? (2) What are the short comings (if any) of US? (3) What lies beyond UGRA? To rationally answer these questions, one must carefully dissect 3 interconnected elements: the documented benefits of US, its limitations, and possible solutions to these shortcomings.
Ultrasound guidance allows the operator to visualize the needle, nerve, unwanted targets (eg, blood vessel and pleura), and spread of local anesthetic agents (LAs).4 In turn, this has facilitated resident training and led to shorter performance and on set times as well as fewer needle passes for single-injection upper extremity blocks. Furthermore, US has been shown to decrease the incidence of hemidiaphragmatic paralysis and pneumothorax for interscalene and supraclavicular blocks, respectively.9
Compared with conventional nerve localization techniques, not only does US increase the success rate of single-injection lower limb blocks while decreasing LA requirement,4 but it also im proves the success of truncal blocks such as rectus sheath 10 and
ilioinguinal/iliohypogastric nerve blocks.11,12 Moreover, randomized trials have demonstrated that, compared to conventional palpation of landmarks, US assistance (ie, preprocedural scanning) results in fewer needle passes/insertions and skin punctures for neuraxial blocks in obstetric and surgical patients. These findings seem most pronounced when expert operators carry out the sono graphic examinations and for patients displaying difficult spinal anatomy.13
Despite the benefits associated with US guidance, proponents of traditional nerve localization techniques could argue that most of these advantages, although statistically significant, may not be clinically relevant in a real-world setting. For instance, for brachial plexus blockade, a decreased onsettime mayor maynotbeimportant, depending on the presence of an induction room. The shorter onset seen with lower extremity blocks becomes trivial if patients also undergogeneral or neuraxial anesthesia intraoperatively. Fur thermore, althoughUSguidance indisputably results infewer nee dle passes for single-injection blocks, this does not translate into a decreased risk of neural injury or increased patient satisfaction. Despite all these arguments, even skeptics cannot overlook or deny the most significant benefit conferred by US guidance: the ability to visualize blood vessels and LA spread decreases the risk of local anesthetic systemic toxicity (LAST). This provides significant advantages for blocks performed by inexperienced opera tors. In a large observational study spanning more than 5 years (2007–2012) and encompassing 20,021 patients undergoing 25,336 peripheral nerve blocks, Barrington and Kluger14 unequivocally demonstrated a lower incidence of LAST with US compared to techniques not utilizing US technology (0.059% vs 0.21%; P = 0.004). The range of point estimates for the odds ratio of LAST with the use of US varied between 0.19 and 0.25.14
In light of the documented benefits derived from US guidance, one could be easily forgiven for overlooking its shortcomings.
However, such drawbacks exist and can be attributed to either the US machine or the operator.15
From a technological standpoint, the decreased US visibility associated with deep neural structures constitutes a challenge. For example, Karmakar et al16 could identify the lumbar plexus in only two-thirds of cases even when using a high-resolution US
machine. As a remedy, these authors combined neurostimulation (NS) and US for lumbar plexus blocks. In teaching centers, primary failure of thoracic epidural blocks represents a significant problem (incidence >20%) and can be partly explained by the difficult anatomy of the thoracic spine.17 Because US assistance con fers benefits for lumbar neuraxial blocks,12 one could hope that it would similarly improve the performance of thoracic epidural blocks. In 70 patients undergoing thoracic or upper abdominal surgery, Auyong et al18 compared conventional palpation of land marks to US preprocedural scanning. These authors found no in tergroup differences in terms of performance times and failure rates (12%–22%). Thus, the inability of US to navigate complex bony structures may constitute yet another limitation. These short comings highlight an interesting paradox inherent to US guid ance: the deeper the neural target, ie, the more one needs US guidance, the less the latter is helpful. Conversely, it becomes most beneficial for superficial targets where its assistance may be less crucial. One need only think of the (superficial) brachial plexus versus the (deep) proximal sciatic nerve in a larger patient. How ever, if history serves as a template, technological limitations may be transient and overcome.19 In other words, with continued progress, clinicians could imagine a not so distant future where in creasingly sophisticated machines and, possibly, 3-dimensional US20 will permit reliable insonation of deep neural structures (eg, the lumbar and sacral plexi),16,21 as well as narrow interlaminar vertebral windows.
In contrast, operator-based shortcomings besetting UGRA stem from obstacles related to perception or interpretation. Thus, they may be time-insensitive. Currently, the greatest strength of US guidance, the operator’s ability to visualize LA spread in relation to the neural target, also masks a twin liability: the definition of neural proximity and its perception by the human eye. Because LAs work by blocking the sodium channels of axons, they must diffuse across neural membranes down a concentration gradient (Fick’s second Law of Diffusion) to reach these target axons. Thus, most anesthesiologists would agree that LA injection should be carried out in close proximity to nerves in an attempt to facili tate transmembrane diffusion.22 However, the ideal distance be tween a nerve and the tip of a needle or catheter has not been established. For instance, Spence et al23 and Palhais et al24 have achieved successful single-injection interscalene blocks by injecting LA immediately next to and 4 mm away from the brachial plexus, respectively. Furthermore, even if the optimal distance between nerve and needle tip could be elucidated for all peripheral nerve blocks, the perception of such contiguity by the naked eye remains subjective and entirely operator dependent.
The micro anatomy and ultra-anatomy of perineural membranes complexify the issue of needle-to-nerve proximity beyond a simple question of physical distance (Fig. 1). The nature of the membrane itself may constitute the key variable. Although descriptions of neural microanatomy have been present in the literature for over a century,25–29 the anesthesiologist’s burgeoning understanding of extraneural tissue layers and the paraneurium (also called the circumneurium) merely dates back to 2012.22,30–35 Only recently have operators realized that the so-called sweet spot of the nerve, described by masters of yester-year, may in fact be synonymous with the subparaneural (ie, subcircumneural) space.22 For example, since 2012, multiple trials have observed that, compared to
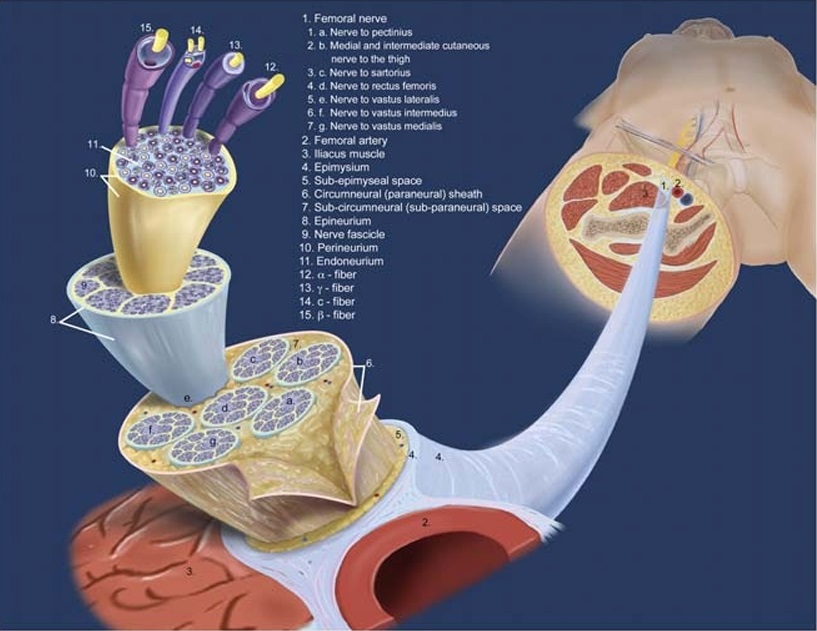
FIGURE 1. Microstructure of the femoral nerve. The femoral nerve is not a single nerve but a bundle of nerves. The branches of the femoral nerve are enclosed in a common circumneural sheath (6; also known as paraneural sheath). Outside the circumneural sheath is the subepimysial space (5), whichis surroundedby the epimysium (4)—the fasciathat surroundsthe nerves, muscles,and bloodvessels. Injection into this space forms the so-called doughnut sign on ultrasound. Deep to the circumneural sheath (6) is the subcircumneural space (7), which is thought to be the ideal space for catheter placement for a CNB. The next layer, which encloses each individual nerve, is the
epineurium (8). In turn, each fascicle (9) is surrounded by its own perineurium (10). Reproduced with kind permission of Mary K. Bryson from The Primer of Regional Anesthesia Anatomy. 2nd ed. Boezaart AP, ed. Kalona, IA: Printer’s Workshop; 2017; RAEducation.com.
the conventional supraparaneural technique, subparaneural LAin jection results in improved efficacy (ie, increased success) and ef ficiency (ie, shorter performance and onset times, as well as fewer needle passes) for popliteal sciatic nerve block.30,36,37 However, in clinical practice, the paraneurium can be difficult to insonate and subparaneural LA injection, a challenge to recognize. These phenomena can be attributed to the current state of “regular,” non–high-definition US machines, as well as subjective percep tion by the human eye. For instance, to detect needle penetration
of the paraneurium-like prevertebral fascia, which surrounds neural clusters in the supraclavicular fossa, operators must often relyon surrogate “pulsatility” of the target cluster: the latter expands concentrically with the quick injection of a small bolus (1 mL)
of LAanddeflates concentrically after said bolus.38–40 Therefore, although our collective understanding of the paraneurium has yet to reach full maturity, and further investigation is very much required, one cannot help but wonder if perhaps our inability to consistently detect subparaneurial LA injection can result in occasional place ment of continuous nerve block (CNB) catheters in the subepimysial (ie, supraparaneural) space instead of the subparaneural space.22 In turn, this may explain instances of secondary block failure de spite the use of USguidanceand,possibly, the negative perception of CNBs by some surgeons.41
Perhaps the greatest source of confusion currently afflicting UGRA originates from an intellectual (mis)interpretation of its application. With the exception of adductor canal blocks,42 ap proaches for brachial plexus, lower limb and common truncal blocks (eg, rectus sheath, ilioinguinal/iliohypogastric and thoracic paravertebral blocks) had been thoroughly described prior to the advent of US.43,44 Anesthesiologists were thus initially content to use US guidance to improve and refine traditional techniques. For in
stance, minimalistic efficiency was sought with the US perivascular technique for axillary blocks45–47 and maximalistic precision, with the targeted intracluster injection technique for supraclavicular blocks.38–40 In 2008, a quiet but seismic shift was unwittingly set in motion with the first description of US-guided subcostal
transversus abdominis plane (TAP) block.48 The latter was initially conceived as a legitimate solution to a real clinical problem: the abdominal wall sensory block provided by the popular US-guided midaxillary TAP block seemed less extensive than the one conferred by its landmark-based counterpart, performed in the triangle of
Petit.49,50 With the new US-guided subcostal method, the needle insertion began near the xiphoid process, and LAwas injected be tween the rectus and transversus abdominis muscles. The description of US-guided subcostal TAP blocks constitutes a pivotal moment in the evolution of UGRA because it provides the first example where US enabled the identification of a target point that wasotherwise inaccessible. In other words, US could now beused to create nerve block techniques de novo. In years that followed, emboldened by the success of subcostal TAP blocks, multiple au thors started using US to identify fascial planes as yet undiscovered by anesthesiologists, where LA could be injected. As a result, an increasing number of “new” US-guidedblocksappeared
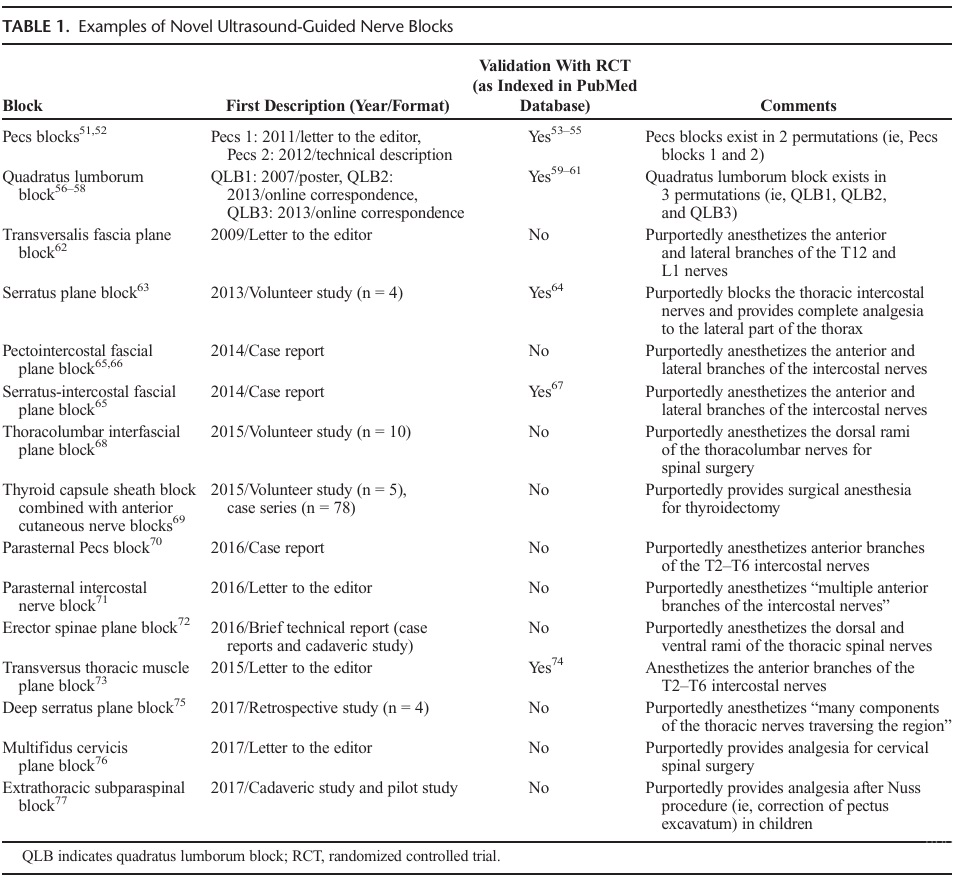
in the literature (Table 1). In many instances, these new nerve blocks were developed prematurely, as their authors, relying on knowledge of sonoanatomy, described a block without a clear application. In contrast, high-yield blocks that have stood the test of time have always been developed in response to a real clinical quandary.78 To further compound the methodological problem, technical validation was usually lacking or confined to anecdotal reports of efficacy (ie, case reports or correspondence) (Table 1). Unfortunately, the latter inherently suffer from selection bias, as reports of inefficacy usually do not get submitted by authors or published by journals. Although some of these new blocks did benefit from cadaveric studies, their authors rarely followed the “proof of concept” dissections with comparative clinical studies or case series large enough to ascertain efficacy, efficiency, and complication rates.
At present, US-guided truncal blocks constitute a wildly hetero geneous mix. Some blocks (such as Pecs and quadratus lumborum blocks) have undergone randomized investigation with trials of var iable quality (Jadad scores raging from 2 to 4).53–55,59–61,64,67,74 Other blocks (such as the pecto-intercostal fascial plane blocks) are still searching for a legitimate application.65,66 A few, such as US-guided thyroid capsular sheath blocks, may never find a clinical role because of sheer interference with the surgical field.69 The fundamental shortcoming afflicting most US- or landmark guided truncal blocks stems from the fact that they do not consis tently address visceral pain, which is mediated by sympathetic (and not somatic) nerves and thus requires concomitant opioid administration (as well as multimodal analgesia). In an era where abuse of prescription opioids has become epidemic,79 patients would perhaps be better served if regional anesthesiology can manage intraoperative and postoperative pain in its entirety, thus avoiding opioid-related adverse effects.80 Furthermore, because all blocks are associated with potential complications, one should prioritize high(est) yield blocks as the dictum “higher the indications, lower the complications” suggests. Therefore, the applicability of many truncal blocks may be limited to minimally-to-moderately painful surgical procedures of the abdominal wall (eg, epigastric/ umbilical/incisional hernia repair) or procedures where the viscera have been completely removed (eg, total abdominal hysterectomy). If significant visceral pain is expected (eg, pancreatic surgery) or early return of bowel function and increased intestinal blood flow are required (eg, colorectal surgery), epidural blockade (and its atten dant sympathetic block) constitutes a logical (and superior) option. AlthoughUSguidancehasindisputably advanced thetechni cal performance of nerve blocks, the intellectual practice of re gional anesthesiology is eerily reminiscent of the one previously encountered with landmark-based techniques. In the days before US, the position of nerves was simply inferred by an appropriate evoked motor response,81 and the initial puncture site was deter mined withcutaneous landmarks. Inevitably, different experts would advocate slightly different sets of landmarks; deep blocks, such as infraclavicular and proximal sciatic blocks, became notorious for the geometric permutations of their landmarks.43,82 Beyond en suring recognition for their authors, these competing sets of land marks did not necessarily improve daily practice and, in fact, only fostered confusion for novices. Unfortunately, the past seems to repeat itself with US descriptions of interfascial planes replacing cutaneous landmarks. However, a saturation point may have been reached, as some authors have started to wonder if all the new truncal blocks are really distinct, and if some do not simply con stitute variations of the same entity.83–86
The limitations associated with UGRA (ie, unreliable insonation of deeper nerves/thoracic interlaminar spaces, ambiguous markers of neural proximity/subparaneural LA injection, and nonvalidated techniques for novel US-guided blocks) may appear daunting. However, careful scrutiny of the literature reveals that most short comings have already been addressed, and in many instances, so
lutions have been proposed.
The inability of US toreliably insonatedeepneuralstructures could be circumvented with adjunctive NS. From a technical standpoint, the concomitant use of US and NS remains controver sial, as it does not always improve block performance. Studies
pertaining to infraclavicular,87,88 axillary,89 and femoral90 blocks have concluded that, compared with US alone, combined US-NS
unnecessarily lengthens the procedural time without increasing success rate. However, the sonographic targets for infraclavicular blocks (6-o’clock position of the axillary artery), the terminal nerves in the axilla, and the femoral nerve are easy to visualize: consequently, the addition of NS to US provides no significant ad vantage for single injection nerve blocks. In contrast, the lumbar and sacral plexi, and the sciatic nerve in some subjects, constitute deeper structures that may benefit from adjunctive NS to confirm the position of the needle/catheter tip.
The high failure rate associated with thoracic epidural blocks in teaching centers cannot be remedied with US preprocedural scanning.17 However, 2 randomized trials have successfully pro posed non-US solutions to the problem. When an epidural needle
(or catheter) is correctly positioned inside the epidural space, pres sure measurement at its tip results in a pulsatile waveform syn chronized with arterial pulsations.91 In a 2-center trial (n = 100), Arnuntasupakul et al92 randomized patients requiring thoracic
epidural blocks to a traditional loss-of-resistance (LOR) technique or LORconfirmed with epiduralwaveform analysis (EWA). These authors found that the latter led to a substantial decrease in the primary failure rate (2% vs 24%; P = 0.002).92 Similarly, Parra et al93 enrolled 100 patients undergoing thoracic epidural blockade and randomized them to a palpation- or fluoroscopy-guided tech nique. Parra et al also observed that fluoroscopy resulted in a lower failure rate (2% vs 26%; P= 0.01). Therefore, the additional equip ment required by EWA and radiation exposure associated with fluoroscopy may be offset by the attendant 10-fold decrease in
primary failure rate. Furthermore, besides improving the success of preoperative thoracic epidural blocks, EWA and fluoroscopy/epidurogram also provide diagnostic versatility, as they can be used in the recovery room to managenonfunctioning epidural cath eters.94,95 Interestingly, adjunctive NS can also be used to confirm correct placement of epidural catheters.96 Although it seems to pro vide high sensitivity and specificity,97 further investigation is re quired to compare conventional and NS-confirmed LOR. Neural proximityand subparaneural LAinjection require ob
jective sonographic signs to ensure interobserver reproducibility. For example, LA injection deep to the paraneural (circumneural) sheath of the sciatic nerve at the neural bifurcation in the popliteal fossa results in a distinctive concentric expansion of the sheath with peripheral sequestration of the tibial and common peroneal nerves.36,98 Similarly, LA injection dorsal to the axillary artery should result in a “double bubble sign” and a “silhouette sign” for single-injection paracoracoid infraclavicular and axillary blocks, respectively.99,100 In some anatomical locations, such as the costo clavicular space,101 it may be feasible to position a CNB catheter where all 3 cords of the brachial plexus are bundled together inside the same plexic sheath.102 Unfortunately, no such US markers of proximity (or subparaneural LA injection) exist for many nerve blocks. For instance, for femoral and proximal (parasacral/ transgluteal) sciatic blocks, the anesthesiologist is often left to de cide when the needle/catheter tip is sufficiently close to the nerve, ie, in the subparaneural (subcircumneural) space. Such a discre
tionary end point may result in different success rates between strict and lax operators, especially for perineural catheters (where LA concentrations are typically dilute, and infusion rates are low). Whereas strict operators would endeavor to place the catheter tip in a subparaneural (subcircumneural) location, their lax counter parts may be satisfied with a simple subepimysial location.22 To prevent such a possibility, the combined use of US-NS (the so called dual technique) could ensure an objective and homoge neousendpoint(ie,the presence ofanevokedmotor response).103
However, in light of an increasingly cost-conscious climate, the authors recommend that each center conduct an internal audit to quantify the secondary failure rate of its US-guided CNB cathe ters. Knowledge of this crucial variable will help determine if op
erators should systematically use stimulating catheters or if the latter should be reserved for specific scenarios (eg, inexperienced operators or deep neural structures such as the lumbar plexus). During these cost projections, anesthesiologists and administra tors should not forget that the expense incurred by stimulating catheter kits is generally dwarfed by the time and cost associated with redoing nonfunctional blocks or readmitting outpatients with suboptimal CNBs. It is also the authors’ personal belief that infra structure, more so than knowledge or technical skills, explains the difference between strict and lax operators: anesthesiologists who work in centers with mature acute pain services or who are pro vided sufficient time to follow their patients postoperatively are usually more inclined to become strict operators.
The solution to nonvalidated US-guided blocks flooding the literature is elegantly simple. In an ideal world, a new nerve block should answer a specific clinical need, and its first description should take the form of an adequately powered, observer-blinded, randomized comparison against the established standard of care or, at the very least, a large case series. To indiscriminately publish every new block technique would promote a recipe-like “this is how I do it” mentality, which constitutes the antithesis of evidence based practice. Yet reporting new innovations only after they have been vetted by clinical trials may delay the anesthesiologist’s de sire to remain on the cutting edge.104 However, case reports and letters to the editor can no longer be the answer. This explains why a journal such as Regional Anesthesia and Pain Medicine christened the section “Brief Technical Report” in 2003 in an at tempt to bridge the gap between anecdotal innovation and ran domized trial.104 Therefore, the collective responsibility to produce science rests not only with investigators but also with peer reviewers and editors alike. More importantly, researchers should not pass upopportunities tovalidate promising US-guidedtechniques even if the latter originated from another center. This collaborative pro cess is best exemplified with the recent investigation of the novel method for US-guided infraclavicular blockade, the costoclavi cular block. The latter was first described in 2015 in a letter to the editor by Karmakar et al.105 The following year, these authors published a Brief Technical Report detailing its anatomical foun dations in cadaveric specimens.101 Subsequently, they carried out a clinical case series in 30 patients (also published as a Brief Technical Report).102 Their preliminary works allowed another group of authors to conduct a randomized trial comparing cos
toclavicular and conventional (paracoracoid) US-guided infra clavicular block,106 as well as a dose-finding trial.107 A quick survey of trial registries reveals that another randomized trial (NCT02657291) is currently underway.
Byenabling the operator to visualize in real time the needle, nerve, and spread of LA, UShasrevolutionized the practice of pe ripheral, neuraxial, and truncal nerve blocks. Going forward, one should ensure that US guidance remains a tool and not a school of regional anesthesiology. In other words, US must not be blindly embraced at the detriment of other valuable modalities such as NS, fluoroscopy, epidurogram, and waveform analysis. The art and the science lie in knowing which tool to use in which setting. Even simple techniques such as the “double pop” fascia iliaca block108 and the transarterial axillary block109 can result in signif icant benefits for patients if judiciously employed in the right con text. Although memorable, the mnemonic“UGRA”mayhaveled many operators astray by focusing exclusively on US guidance. New US-guided blocks could and should still be developed as long as scientific reputation does not supersede science, and eponymity remains subservient to evidence, as well as definable clinical needs. The description of the novel blocks made possible by UScertainly represents an exciting time in the evolution of an esthesiology, but one need not (and should not) abandon suc cesses of the past simply because a new tool graces the arsenal.
In hindsight, future pundits will likely refer to current times as the age of innocence. Perhaps, as a collective of like-minded colleagues, we should now transition to ourage of reason.Anep och where patient care drives technology (instead of the opposite scenario), where adding value to the perioperative surgical home becomes the common goal,110 and where new blocks, adequately vetted and compared against the criterion standard (instead of placebo), are developed to fill real-world clinical gaps. Thus, to para phrase Seattle’s favorite adopted son, Lee Jun-Fan (also known as BruceLee),thespecialtyshould not consist of a daily increase, but a daily decrease: one should hack away incessantly at the inessentials. Ultimately, to the question, “What lies beyond UGRA?” there can be only one true answer: “what came before UGRA: the patient.”
1. Kapral S, Krafft P, Eibenberger K, Fitzgerald R, Gosch M, Weinstabl C.
Ultrasound-guided supraclavicular approach for regional anesthesia of the
brachial plexus. Anesth Analg. 1994;78:507–513.
2. Tran DQ, Muñoz L, Russo G, Finlayson RJ. Ultrasonography and
stimulating perineural catheters for nerve blocks: a reviewof the evidence.
Can JAnaesth. 2008;55:447–457.
3. Boezaart AP, Ihnatsenka BV. Cervical paravertebral block for elbow and
wrist surgery: the jury verdict may be neither easy, nor popular.
Reg Anesth Pain Med. 2014;39:361–362.
4. Neal JM, Brull R, Horn JL, et al. The Second American Society of
Regional Anesthesia and Pain Medicine evidence-based medicine
assessment of ultrasound-guided regional anesthesia: executive summary.
Reg Anesth Pain Med. 2016;41:181–194.
5. Sites BD, Chan VW, Neal JM, et al. The American Society of regional
Anesthesia and Pain Medicine and the European Society of Regional
Anesthesia and Pain Therapy joint committee recommendations for
education and training in ultrasound-guided regional anesthesia.
Reg Anesth Pain Med. 2009;34:40–46.
6. Davis JJ, Swenson JD, Greis PE, Burks RT, Tashjian RZ. Interscalene
block for postoperative analgesia using only ultrasound guidance: the
outcome in 200 patients. JClinAnesth. 2009;21:272–277.
7. Swenson JD. Resident training for peripheral nerve blockade should be
conducted solely with ultrasound. ASRA News.2009:10–11.
8. Bøtker MT, Vang ML, Grøfte T, Kirkegaard H, Frederiksen CA, Sloth E.
Implementing point-of-care ultrasonography of the heart and lungs in
an anesthesia department. Acta Anaesthesiol Scand. 2017;61:156–165.
9. Neal JM. Ultrasound-guided regional anesthesia and patient safety:
update of an evidence-based analysis. Reg Anesth Pain Med.
2016;41:195–204.
10. Dolan J, Lucie P, Geary T, Smith M, Kenny GN. The rectus sheath
block: accuracy of local anesthetic placement by trainee anesthesiologists
using loss of resistance or ultrasound guidance. Reg Anesth Pain Med.
2009;34:247–250.
11. Willschke H, Marhofer P, Bösenberg A, et al. Ultrasonography for
ilioinguinal/iliohypogastric nerve blocks in children. Br J Anaesth
2005;95:226–230.
12. Weintraud M, Lundblad M, Kettner SC, et al. Ultrasound versus
landmark-based technique for ilioinguinal-iliohypogastric nerve blockade
in children: the implications on plasma levels of ropivacaine. Anesth
Analg 2009;108:1488–1492.
13. Elgueta MF, Duong S, Finlayson RJ, Tran Q. Ultrasonography for
neuraxial blocks: a review of the evidence. Minerva Anestesiol.
2017;83:512–523.
14. Barrington MJ, Kluger R. Ultrasound guidance reduces the risk of local
anesthetic systemic toxicity following peripheral nerve blockade.
RegAnesthPainMed. 2013;38:289–299.
15. Abdallah FW, MacFarlane AJ, Brull R. The requisites of needle-to-nerve
proximity for ultrasound-guided regional anesthesia: a scoping review
of the evidence. RegAnesthPainMed. 2016;41:221–228.
16. Karmakar MK, Li JW, Kwok WH, Hadzic A. Ultrasound-guided lumbar
plexus block using a transverse scan through the lumbar intertransverse
space: a prospective case series. RegAnesthPainMed.2015;40:75–81.
17. Tran DQ, van Zundert TC, Aliste J, Engsusophon P, Finlayson RJ.
Primary failure of thoracic epidural analgesia in training centers: the
invisible elephant? RegAnesthPainMed. 2016;41:309–313.
18. Auyong DB, Hostetter L, Yuan SC, Slee AE, Hanson NA. Evaluation of
ultrasound-assisted thoracic epidural placement in patients undergoing
upper abdominal and thoracic surgery: a randomized, double-blind study.
RegAnesthPainMed. 2017;42:204–209.
19. Choquet O, Abbal B, Capdevila X. The new technological trends in
ultrasound-guided regional anesthesia. Curr Opin Anaesthesiol.
2013;26:605–612.
20. Choquet O, Capdevila X. Case report: three-dimensional high-resolution
ultrasound-guided nerve blocks: a new panoramic vision of local
anesthetic spread and perineural catheter tip location. Anesth Analg.
2013;116:1176–1181.
21. BendtsenTF,LönnqvistPA,Jepsen KV,PetersenM,KnudsenL,Børglum
J. Preliminary results of a new ultrasound-guided approach to block the
sacral plexus: the parasacral parallel shift. Br J Anaesth. 2011;107:
278–280.
22. Boezaart AP. The sweet spot of the nerve: is the “paraneural sheath”
named correctly, and does it matter? Reg Anesth Pain Med.2014;39:
557–558.
23. Spence BC, Beach ML, Gallagher JD, Sites BD. Ultrasound-guided
interscalene blocks: understanding where to inject the local anaesthetic.
Anaesthesia. 2011;66:509–514.
24. Palhais N, Brull R, Kern C, et al. Extrafascial injection for interscalene
brachial plexus block reduces respiratory complications compared with
a conventional intrafascial injection: a randomized, controlled,
double-blind trial. Br J Anaesth. 2016;116:531–537.
25. Key A, Retzius G. Studies in the Anatomy of the Nervous System and
Connective Tissue. Samson and Wallin: Stockholm, Sweden; 1876.
26. Gordinier HC. The Gross and Minute Anatomy of the Central Nervous
System. Philadelphia, PA: Blackiston’s Son & Co; 1899.
27. Moore DC, Hain RF, Ward A, Bridenbaugh LD Jr. Importance of the
perineural spaces in nerve blocking. J Am Med Assoc. 1954;156:1050–1053.
28. Selander D, Sjöstrand J. Longitudinal spread of intraneurally injected local
anesthetics. An experimental study of the initial neural distribution following
intraneural injections. Acta Anaesthesiol Scand. 1978;22:622–634.
29. Millesi H, Zoch G, Rath T. The gliding apparatus of peripheral nerve
and its clinical significance. AnnChirMainMembSuper. 1990;9:87–97.
30. Andersen HL, Andersen SL, Tranum-Jensen J. Injection inside the
paraneural sheath of the sciatic nerve: direct comparison among
ultrasound imaging, macroscopic anatomy, and histologic analysis.
RegAnesthPainMed. 2012;37:410–414.
31. Karmakar MK, Shariat AN, Pangthipampai P, Chen J. High-definition
ultrasound imaging defines the paraneural sheath and the fascial
compartments surrounding the sciatic nerve at the popliteal fossa.
RegAnesthPainMed. 2013;38:447–451.
32. Reina MA, Sala-Blanch X. Cross-sectional microscopic anatomy of the
brachial plexus and paraneural sheaths. In: Reina MA, ed. Atlas of
Functional Anatomy for Regional Anesthesia and Pain Medicine.
New York, NY: Springer; 2015.
33. Boezaart AP. Microanatomy of the sciatic nerve. In: Boezaart AP,
ed. The Anatomical Foundations of Regional Anesthesia.Sharjah,United
Arab Emirates: Bentham Science Publishers; 2016.
34. Boezaart AP. The microanatomy of the brachial plexus and peripheral
nerves. In: Boezaart AP, ed. The Anatomical Foundations of Regional
Anesthesia. Sharjah, United Arab Emirates: Bentham Science Publishers; 2016.
35. Franco CD. Connective tissues associated with peripheral nerves.
RegAnesthPainMed. 2012;37:363–365.
36. Tran DQ, Dugani S, Pham K, Al-Shaafi A, Finlayson RJ. A randomized
comparison between subepineural and conventional ultrasound-guided
popliteal sciatic nerve block. RegAnesthPainMed. 2011;36:548–552.
37. Perlas A, Wong P, Abdallah F, Hazrati LN, Tse C, Chan V.
Ultrasound-guided popliteal block through a common paraneural sheath
versus conventional injection: a prospective, randomized, double-blind
study. Reg Anesth Pain Med. 2013;38:218–225.
38. Techasuk W, González AP, Bernucci F, Cupido T, Finlayson RJ, Tran DQ.
Arandomized comparison between double-injection and targeted
intracluster injection ultrasound-guided supraclavicular brachial plexus
block. Anesth Analg. 2014;118:1363–1369.
39. Yazer MS, Finlayson RJ, Tran DQ. A randomized comparison between
infraclavicular block and targeted intracluster injection supraclavicular
block. RegAnesthPainMed.2015;40:11–15.
40. Arnuntasupakul V, Leurcharusmee P, Chora de la Garza D, Ah-Kye S,
Finlayson RJ, Tran DQ. A randomized trial comparing axillary block
versus targeted intracluster injection supraclavicular block for upper
limb surgery. Can J Anaesth. 2015;62:1287–1294.
41. Boezaart AP, Zasimovich Y. Why continuous nerve blocks fail.
Techniques Orthop.2017.
42. Wong WY, Bjørn S, Strid JM, Børglum J, Bendtsen TF. Defining the
location of the adductor canal using ultrasound. Reg Anesth Pain Med.
2017;42:241–245.
43. Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: a
review of approaches and techniques. Can J Anaesth. 2007;54:662–674.
44. Tran DQ, Clemente A, Finlayson RJ. A review of approaches and techniques
for lower extremity nerve blocks. Can J Anaesth.2007;54:922–934.
45. Bernucci F, González AP, Finlayson RJ, Tran DQ. A prospective,
randomized comparison between perivascular and perineural
ultrasound-guided axillary brachial plexus block. RegAnesthPainMed.
2012;37:473–477.
46. Sites BD, Neal JM. Keep it simple. RegAnesthPainMed.2012;37:
465–466.
47. Neal JM, Gerancher JC, Hebl JR, et al. Upper extremity regional
anesthesia: essentials of our current understanding, 2008. Reg Anesth
Pain Med. 2009;34:134–170.
48. Hebbard P. Subcostal transversus abdominis plane block under
ultrasound guidance. Anesth Analg. 2008;106:674–675.
49. Tran TM, Ivanusic JJ, Hebbard P, Barrington MJ. Determination of
spread of injectate after ultrasound-guided transversus abdominis plane
block: a cadaveric study. Br J Anaesth. 2009;102:123–127.
50. Rafi AN. Abdominal field block: a new approach via the lumbar triangle.
Anaesthesia. 2001;56:1024–1026.
51. Blanco R. The ‘Pecs block’: a novel technique for providing analgesia
after breast surgery. Anaesthesia. 2011;66:847–848.
52. Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of
Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp
Anestesiol Reanim. 2012;59:470–475.
53. Kulhari S, Bharti N, Bala I, Arora S, Singh G. Efficacy of pectoral
nerve block versus thoracic paravertebral block for postoperative
analgesia after radical mastectomy: a randomized controlled trial.
Br JAnaesth. 2016;117:382–386.
54. Hetta DF, Rezk KM. Pectoralis-serratus interfascial plane block vs
thoracic paravertebral block for unilateral radical mastectomy with
axillary evacuation. JClinAnesth.2016;34:91–97.
55. Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in
multimodal analgesia for breast cancer surgery: a randomized clinical
trial. RegAnesthPainMed.2015;40:68–74.
56. Blanco R. TAP block under ultrasound guidance: the description of a “no
pops” technique: 271. RegAnesthPainMed. 2007;32(suppl 15):S1–S130.
57. Blanco R, McDonnell JG. Optimal point of injection: the quadratus
lumborum type I and II blocks. 2013. Available at: http://www.
respond2articles.com/ANA/forums/post/1550.aspx. Accessed April 27, 2017.
58. Børglum J, Jensen K, Moriggl B, et al. Ultrasound-guided transmuscular
quadratus lumborum blockade. BJA Out Blue e-Letters.2013.
59. Blanco R, Ansari T, Girgis E. Quadratus lumborum block for
postoperative pain after caesarean section: a randomised controlled trial.
Eur J Anaesthesiol. 2015;32:812–818.
60. BlancoR,Ansari T, Riad W, ShettyN. Quadratus lumborum block versus
transversus abdominis plane block for postoperative pain after cesarean
delivery: a randomized controlled trial. Reg Anesth Pain Med.2016;41:
757–762.
61. Carline L, McLeod GA, Lamb C. A cadaver study comparing spread of
dye and nerve involvement after three different quadratus lumborum
blocks. Br J Anaesth. 2016;117:387–394.
62. Hebbard PD. Transversalis fascia plane block, a novel ultrasound-guided
abdominal wall nerve block. Can J Anaesth. 2009;56:618–620.
63. Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block:
a novel ultrasound-guided thoracic wall nerve block. Anaesthesia.
2013;68:1107–1113.
64. KhalilAE,AbdallahNM,BashandyGM,KaddahTA.Ultrasound-guided
serratus anterior plane block versus thoracic epidural analgesia for
thoracotomy pain. J Cardiothorac Vasc Anesth. 2017;31:152–158.
65. López-Matamala B, Fajardo M, Estébanez-Montiel B, Blancas R,
Alfaro P, Chana M. A new thoracic interfascial plane block as anesthesia
for difficult weaning due to ribcage pain in critically ill patients.
Med Intensiva. 2014;38:463–465.
66. De la Torre PA, García PD, Alvarez SL, Miguel FJ, Pérez MF. A novel
ultrasound-guided block: a promising alternative for breast analgesia.
Aesthet Surg J. 2014;34:198–200.
67. Kim JS, Lee J, Soh EY, et al. Analgesic effects of ultrasound-guided
serratus-intercostal plane block and ultrasound-guided intermediate
cervical plexus block after single-incision transaxillary robotic
thyroidectomy: a prospective, randomized, controlled trial. Reg Anesth
Pain Med. 2016;41:584–588.
68. HandWR,TaylorJM,HarveyNR,etal.Thoracolumbarinterfascial plane
(TLIP) block: a pilot study in volunteers. Can J Anaesth 2015;62:
1196–1200.
69. WangQ,LiZ,XuS,etal.Feasibilityofultrasound-guided capsule-sheath
space block combined with anterior cervical cutaneous nerves block
for thyroidectomy: an observational pilot study. BMC Anesthesiol.
2015;15:4.
70. Hansen CK, Dam M, Poulson TD, Loonqvist PA, Bendsten TF,
Borglum J. Ultrasound-guided parasternal Pecs block: a new and useful
supplement to Pecs 1 and serratus anterior plane blocks. Anaesthesia Cases.
Available at: http://www.anaesthesiacases.org/cases-reports/2016-0007.
71. Ohgoshi Y, Ino K, Matsukawa M. Ultrasound-guided parasternal
intercostal nerve block. JAnesth. 2016;30:916.
72. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae
plane block: a novel analgesic technique in thoracic neuropathic pain.
RegAnesthPainMed. 2016;41:621–627.
73. Ueshima H, Kitamura A. Blocking of multiple anterior branches of
intercostal nerves (Th2-6) using a transversus thoracic muscle plane
block. RegAnesthPainMed. 2015;40:388.
74. Ueshima H, Otake H. Addition of transversus thoracic muscle plane
block to pectoral nerves block provides more effective perioperative
pain relief than pectoral nerves block alone for breast cancer surgery.
Br JAnaesth. 2017;118:439–443.
75. Piracha MM, Thorp SL, Puttanniah V, Gulati A. A “tale of two planes”:
deep versus superficial serratus plane block for postmastectomy pain
syndrome. RegAnesthPainMed. 2017;42:259–262.
76. Ohgoshi Y, Izawa H, Kori S, Matsukawa M. Multifidus cervicis plane
block is effective for cervical spine surgery. Can J Anaesth.2017;64:
329–330.
77. Bryskin RB, Robie DK, Mansfield FM, Freid EB, Sukumvanich S.
Introduction of a novel ultrasound-guided extrathoracic sub-paraspinal
block for control of perioperative pain in NUSS procedure patients.
J Pediatr Surg. 2017;52:484–491.
78. Boezaart AP, de Beer JF, Du Toit C, van Rooyen K. A new technique of
continuous interscalene nerve block. Can J Anaesth. 1999;46:275–281.
79. Kotecha MK, Sites BD. Pain policy and abuse of prescription opioids in
the USA: a cautionary tale for Europe. Anaesthesia. 2013;68:1210–1215.
80. Boezaart AP, Wright T. Rational use and pitfalls of regional anesthesia
for orthopaedic surgery. Techniques Orthop. (Accepted) 2017.
81. De Andrés J, Sala-Blanch X. Peripheral nerve stimulation in the
practice of brachial plexus anesthesia: a review. RegAnesthPainMed.
2001;26:478–483.
82. Franco CD. Posterior approach to the sciatic nerve in adults: is euclidean
geometry still necessary? Anesthesiology. 2003;98:723–728.
83. Del Buono R, Costa F, Agrò FE. Parasternal, pecto-intercostal, Pecs,
and transverse thoracic muscle plane blocks: a rose by any other name
would smell as sweet. Reg Anesth Pain Med. 2016;41:791–792.
84. Ueshima H, Otake H. Similarities between the retrolaminar and erector
spinae plane blocks. RegAnesthPainMed. 2017;42:123–124.
85. Murouchi T. Consideration of block nomenclature: erector spinae plane
block or retrolaminar block? RegAnesthPainMed. 2017;42:124.
86. Chin KJ, McDonnell JG, Carvalho B, Sharkey A, Pawa A, Gadsden J.
Essentials of our current understanding: abdominal wall blocks.
RegAnesthPainMed. 2017;42:133–183.
87. Dingemans E, Williams SR, Arcand G, et al. Neurostimulation in
ultrasound-guided infraclavicular block: a prospective randomized trial.
Anesth Analg 2007;104:1275–1280.
88. Gurkan Y, Tekin M, Acar S, Solak M, Toker K. Is nerve stimulation
needed during an ultrasound-guided lateral sagittal infraclavicular block?
Acta Anaesthesiol Scand. 2010;54:403–407.
89. Chan VW, Perlas A, McCartney CJ, Brull R, Xu D, Abbas S. Ultrasound
guidance improves success rate of axillary brachial plexus block.
Can JAnaesth. 2007;54:176–182.
90. SitesBD,BeachML,ChinnCD,RedborgKE,GallagherJD.A
comparison of sensory and motor loss after a femoral nerve block
conducted with ultrasound versus ultrasound and nerve stimulation.
RegAnesthPainMed. 2009;34:508–513.
91. Tran DQ, González AP, Bernucci F, Finlayson RJ. Confirmation of
loss-of-resistance for epidural analgesia. RegAnesthPainMed.
2015;40:166–173.
92. Arnuntasupakul V, van Zundert TC, Vijitpavan A, et al. A randomized
comparison between conventional and waveform-confirmed
loss-of-resistance for thoracic epidural blocks. Reg Anesth Pain Med.
2016;41:368–373.
93. Parra MC, Washburn K, Brown JR, et al. Fluoroscopy guidance
increases the incidence of thoracic epidural catheter placement within
the epidural space: a randomized trial. RegAnesthPainMed.
2017;42:17–24.
94. Ghia JN, Arora SK, Castillo M, Mukherji SK. Confirmation of
location of epidural catheters by epidural pressure waveform and
computed tomography cathetergram. RegAnesthPainMed.
2001;26:337–341.
95. Porteous GH, Neal JM, Slee A, Schmidt H, Low DE. A standardized
anesthetic and surgical clinical pathway for esophageal resection: impact on
length of stay and major outcomes. RegAnesthPainMed. 2015;40:
139–149.
96. Tsui BC, Gupta S, Finucane B. Confirmation of epidural catheter
placement using nerve stimulation. Can J Anaesth. 1998;45:640–644.
97. Charghi R, Chan SY, Kardash KJ, Finlayson RJ, Tran DQ. Electrical
stimulation of the epidural space using a catheter with a removable stylet.
RegAnesthPainMed. 2007;32:152–156.
98. Tiyaprasertkul W, Bernucci F, Gonzalez A, et al. A randomized
comparison between single- and triple-injection subparaneural popliteal
sciatic nerve block. RegAnesthPainMed. 2015;40:315–320.
99. Tran DQ, Charghi R, Finlayson RJ. The “double bubble” sign for
successful infraclavicular brachial plexus blockade. Anesth Analg.
2006;103:1048–1049.
100. Tran DQ, Pham K, Dugani S, Finlayson RJ. A prospective, randomized
comparison between double-, triple- and quadruple-injection
ultrasound-guided axillary brachial plexus block. RegAnesthPainMed.
2012;37:248–253.
101. Sala-Blanch X, Reina MA, Pangthipampai P, Karmakar MK. Anatomic
basis for brachial plexus block at the costoclavicular space: a cadaver
anatomic study. Reg Anesth Pain Med. 2016;41:387–391.
102. Li JW, Songthamwat B, Samy W, Sala-Blanch X, Karmakar MK.
Ultrasound-guided costoclavicular brachial plexus block: sonoanatomy,
technique, and block dynamics. Reg Anesth Pain Med.2017;42:233–240.
103. BoezaartAP,DavisG,Le-WendlingL.Recoveryafterorthopedicsurgery:
techniques to increase duration of pain control. Curr Opin Anaesthesiol.
2012;25:665–672.
104. Neal JM. Between innovation and proven value: achieving a balance in
technical reporting. RegAnesthPainMed. 2003;28:170–171.
105. Karmakar MK, Sala-Blanch X, Songthamwat B, Tsui BC. Benefits of
the costoclavicular space for ultrasound-guided infraclavicular brachial
plexus block: description of a costoclavicular approach. Reg Anesth
Pain Med. 2015;40:287–288.
106. Leurcharusmee P, Elgueta MF, Tiyaprasertkul W, et al. A randomized
comparison between costoclavicular and paracoracoid ultrasound-guided
infraclavicular block for upper limb surgery. Can J Anaesth.2017;64:
617–625.
107. Sotthisopha T, Elgueta MF, Samerchua A, et al. Minimum effective
volume of lidocaine for ultrasound-guided costoclavicular block.
Reg Anesth Pain Med. 2017;42:571–574.
108. McRaePJ, Bendall JC, Madigan V, Middleton PM. Paramedic-performed
fascia iliaca compartment block for femoral fractures: a controlled trial.
JEmergMed. 2015;48:581–589.
109. MacKay CA, BowdenDF. Axillary brachial plexus block—an underused
technique in the accident and emergency department. JAccidEmergMed.
1997;14:226–229.
110. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as
a future perioperative practice model. Anesth Analg 2014;118:1126–1130.
