Abstract
Background and objectives In 2011, chronic shoulder joint pain was reported by 18.7million Americans. Image-guided radiofrequency ablation has emerged as an alternative intervention to manage chronic shoulder joint pain. To optimize the effectiveness of shoulder denervation, it requires a detailed understanding of the nerve supply to the glenohumeral and acromioclavicular joints relative to landmarks visible
with image guidance. The purpose of this cadaveric study was to determine the origin, course, relationships to bony landmarks, and frequency of articular branches innervating the glenohumeral and acromioclavicular joints.
Methods Fifteen cadaveric specimens were meticulously dissected. The origin, course, and termination of articular branches supplying the glenohumeral and acromioclavicular joints were documented. The frequency of each branch was determined and used to generate a frequency map that included their relationships to bony and soft tissue landmarks.
Results In all specimens, the posterosuperior quadrant of the glenohumeral joint was supplied by suprascapular nerve; posteroinferior by posterior division of axillary nerve; anterosuperior by superior nerve to subscapularis; and anteroinferior by main trunk of axillary nerve. Less frequent innervation was found from lateral pectoral nerve and posterior cord. The acromioclavicular joint was found to be innervated by the lateral pectoral and acromial branch of suprascapular nerves in all specimens. Bony and soft tissue landmarks were identified to localize each nerve.
Conclusions The frequency map of the articular branches supplying the glenohumeral and acromioclavicular joints, as well as their relationship to bony and soft tissue landmarks, provide an anatomical foundation to develop novel shoulder denervation and perioperative pain management protocols.
In 2011, chronic shoulder joint pain was self-reported by 18.7 million Americans. Osteoarthritis is a common cause of chronic shoulder pain and patients who experience moderate to severe symptoms are considered for shoulder arthroplasty. In the USA, 66 995 shoulder replacements were performed in 2011, with the number of procedures projected to increase exponentially. However, it has been reported that up to 22% of patients experience persistent pain following successful shoulder arthroplasty.
Radiofrequency ablation (RFA) has emerged as an alternative intervention to manage chronic shoulder pain. To optimize the effectiveness of shoulder denervation, it is important to precisely localize and target articular branches supplying the glenohumeral joints (GHJ) and acromioclavicular joints (ACJ). Previous anatomical studies, summarized in table, have identified several sources of innervation of the GHJ and/or ACJ, including suprascapular nerves (SSN), lateral pectoral nerves (LPN), and axillary nerves (AN), as well as the nerves to subscapularis (NS). Of the 10 studies found, the innervation of GHJ was reported in nine and that of the ACJ in four (table 1). The frequency of each nerve was seldom reported, and the only comprehensive study was published by Gardner who reported findings with hand-drawn illustrations.
Further investigation of the innervation of GHJ and ACJ relative to bony and soft tissue landmarks identifiable with ultrasound/fluoroscopy are needed, as presently, in the RFA literature the main target has been the SSN. Therefore, the aims of this cadaveric study were to document the origin and frequency of articular branches innervating the GHJ and/or ACJ; trace the course and capsular distribution of each articular branch; and define bony and soft tissue landmarks to localize the articular branches.
Ten formalin and five lightly embalmed cadaveric specimens were used in this study. Due to lack of sufficient data for the purposes of quantitative analysis, sample size calculation was not possible. The specimens had no visible evidence of pathology,
previous surgery, or trauma. SSN, LPN, NS, and AN were meticulously dissected using a 3.5× magnification lens. The nerves were dissected from proximal to distal, beginning at their point of origin at the brachial plexus and continued to their termination. All specimens were photographed. Articular branches terminating in the capsule of GHJ and/or ACJ were documented and their trajectory mapped.
The SSN was traced distally to the suprascapular notch, where it passed inferior to the transverse scapular ligament to lie on the floor of the supraspinous fossa. The medial and lateral trunks of SSN were identified, and any branches were followed to their termination, in short segments, by removing
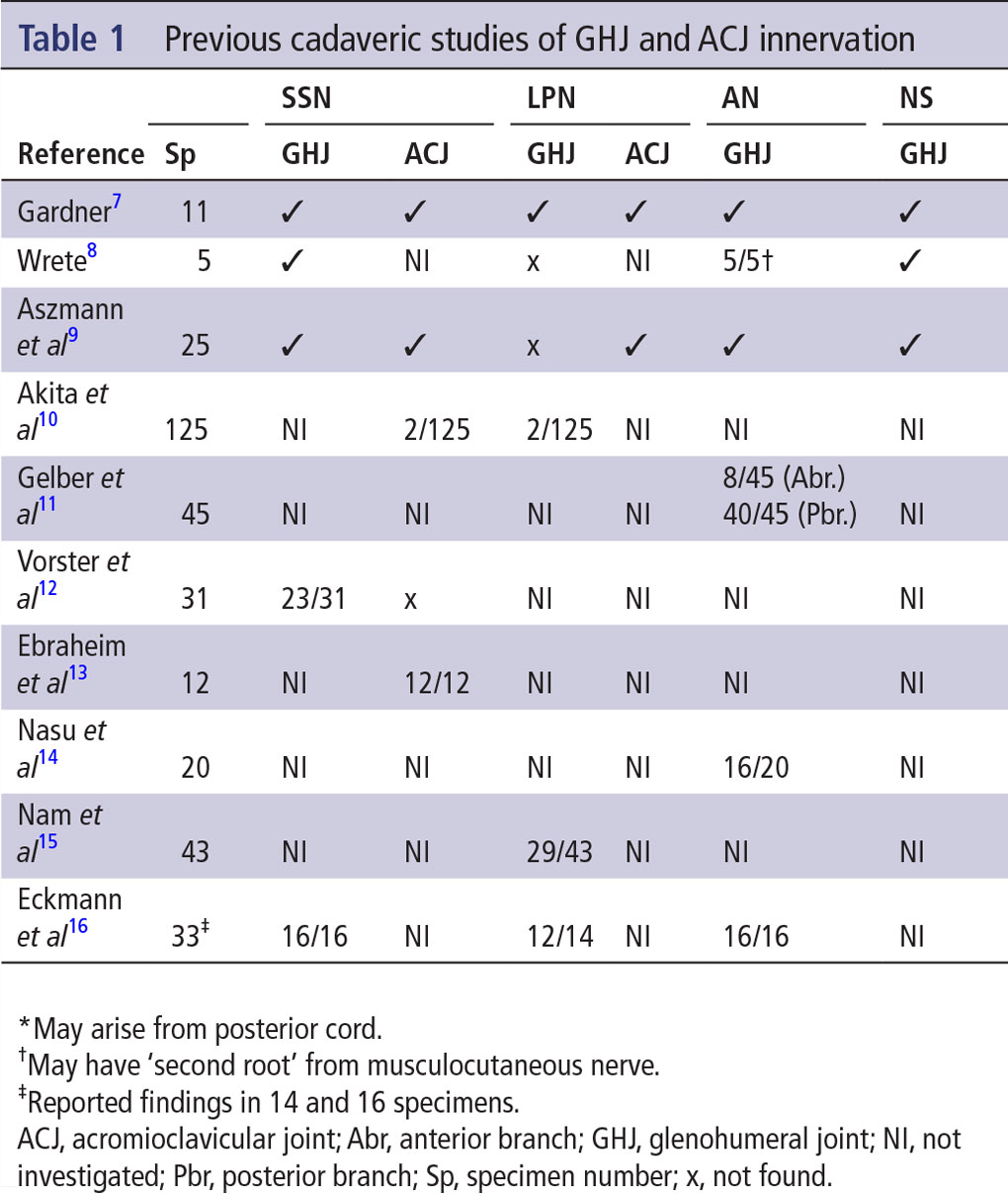
muscle fiber bundles from the overlying supraspinatus. The lateral trunk was followed along the floor of the supraspinous fossa, around the spinoglenoid notch into the infraspinous fossa. Next, the infraspinatus was dissected at the fiber bundle level to expose branches of the lateral trunk as it coursed in the infraspinous fossa. To further document the location of the origins of the medial trunk of SSN and first articular branch from the lateral trunk of SSN, the following methodology was carried out in each dissected specimen (figure 1): midpoints of the suprascapular and spinoglenoid notches were identified; linear distance between the midpoints of the notches was measured using calipers; midpoint of the line quantified in two was demarcated on each specimen with a point using a thin marker; and origins of the medial trunk of SSN and the first articular branch from the lateral trunk of SSN, relative to the midpoint of the line defined in 3, was quantified using calipers.
The LPN was traced from the lateral cord of the brachial plexus to the deltopectoral triangle. The branches of the thoracoacromial artery and vein were removed to expose the branches of LPN. Each nerve branch was followed to its termination in muscle or the joint capsule. The NS were identified at their origin from the posterior cord of the brachial plexus. Each of the nerves was traced to its termination by removing fiber bundles of subscapularis. Motor branches to subscapularis and sensory branches innervating the joint capsule were identified. The AN was traced from the posterior cord through the quadrilateral space with the posterior circumflex humeral artery and veins. The artery and veins were separated from the AN and subsequently removed, while maintaining the integrity of any branches. Branches of AN were followed to their termination and documented.
A frequency map of the innervation of the shoulder joint capsule was generated by consolidating the innervation patterns from each specimen onto a three-dimensional (3D) CT reconstruction of the scapula, humerus and clavicle (Amira for Life Sciences, Thermo Scientific, Waltham, Massachusetts, USA; Maya 2016, Autodesk, San Rafael, California, USA; Paint.Net, dotPDN LLC, Redmond, Washington, USA). The capsular distribution of the articular branches was documented and compared between specimens. Based on the dissected specimens and frequency map, bony and soft tissue landmarks to localize the articular branches were identified.
The GHJ was found to be most consistently innervated by articular branches from SSN, NS, and AN, whereas the ACJ was supplied by LPN and acromial branch from SSN.
Glenohumeral joint
To provide a summary of the innervation of GHJ, the capsule was divided into quadrants: posterosuperior, posteroinferior, anterosuperior, and anteroinferior (figure 2).
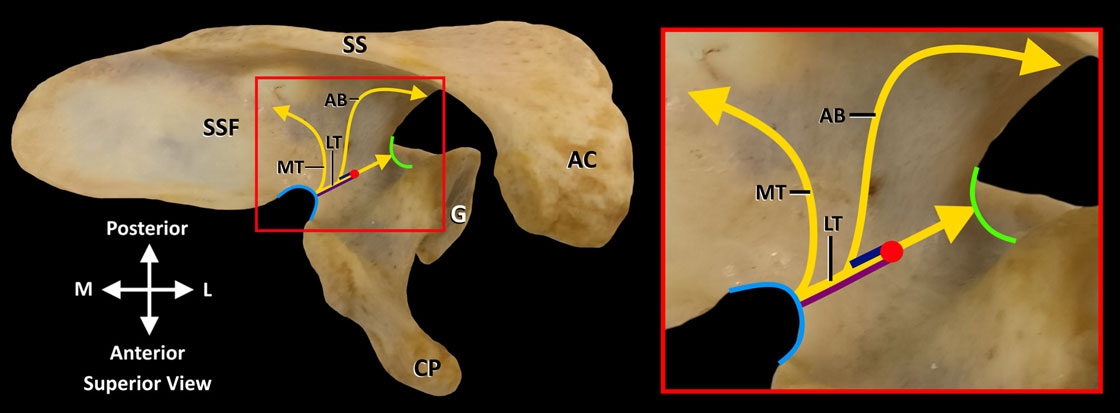
Figure 1 Distance measurements of the MT (purple line) and first AB (dark blue line) of SSN relative to the midpoint (red dot) of a line between the suprascapular (light blue curve) and spinoglenoid (green curve) notches. AB is the first articular branch from LT of SSN. AB, articular branch; AC, acromion process; CP, coracoid process; G, glenoid fossa; LT, lateral trunk; MT, medial trunk; SS, spine of scapula; SSF, supraspinous fossa; SSN, suprascapular nerve. Reprinted with permission from Philip Peng Educational Series
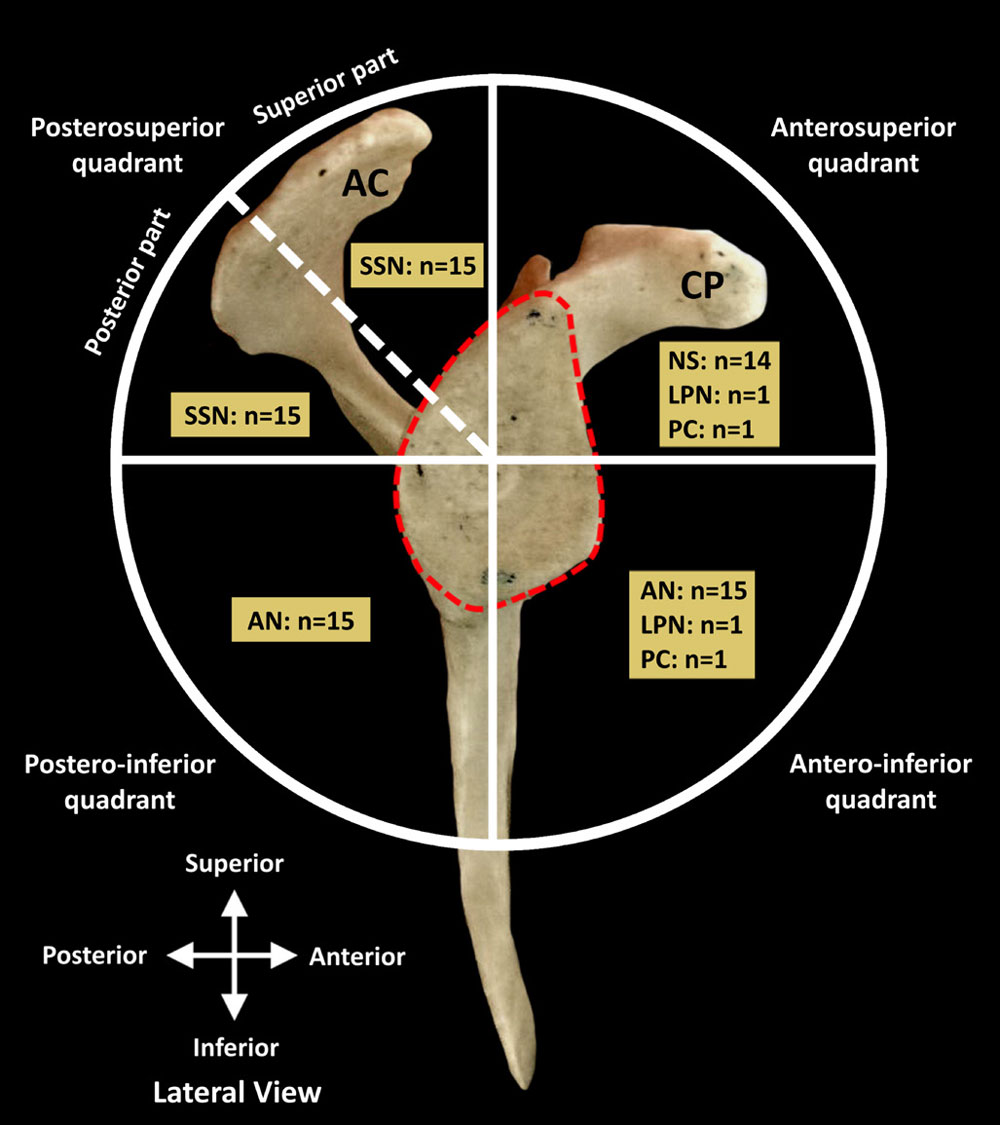
Figure 2 Innervation of quadrants of glenohumeral joint capsule. AC, acromion process; AN, axillary nerve; CP, coracoid process; LPN, lateral pectoral nerve; NS, nerves to subscapularis; PC, posterior cord; SSN, suprascapular nerve. Red dash lines indicate outline of glenoid fossa. Reprinted with permission from Philip Peng Educational Series.
Figure 3 Innervation of ACJ and posterosuperior quadrant of GHJ. (A and B) Articular branches to GHJ originating from the lateral trunk of SSN and acromial branch (br) of SSN and LPN to ACJ. Note the clavicle has been disarticulated and removed in A. (C) SSN articular branches to GHJ and ACJ. Articular branches to GHJ originate from bifurcation of SSN into medial and lateral trunks and acromial branches to ACJ from SSN. AC, acromion process; ACJ, acromioclavicular joint; CL, clavicle; CP, coracoid process; GHJ, glenohumeral joint; HH, humeral head; LPN, lateral pectoral nerve; SS, spine of scapula; SSF, supraspinous fossa; SSN, main trunk of suprascapular nerve; black arrow heads, medial trunk of SSN; *, transverse scapular ligament. Reprinted with permission from Philip Peng Educational Series
Posterosuperior quadrant
The posterosuperior quadrant was innervated by SSN in all 15 specimens. The SSN, after giving off the acromial branch, passed deep to the transverse scapular ligament and immediately divided into medial and lateral trunks (figure 3A). The mean distance of the bifurcation of SSN, from the midpoint of a line connecting the suprascapular and spinoglenoid notches, was 1.07±0.14 cm. Branches from the medial trunk innervated
the anterior region of supraspinatus; no articular branches were found. The lateral trunk coursed along the floor of the supraspinous fossa to the spinoglenoid notch, with the suprascapular artery, supplying the posterior region of supraspinatus and GHJ capsule. In 13 specimens, the GHJ capsule was innervated by articular branches that originated from the lateral trunk starting at the midpoint between the suprascapular notch and the spinoglenoid notch (figure 3A,B). The mean distance of the origin of the first articular branch from the lateral trunk of SSN, from the midpoint of a line connecting the suprascapular and spinoglenoid notches, was 0.41±0.18 cm. In two specimens, the articular branches originated from the bifurcation of the SSN into the medial and lateral trunks (figure 3C). Additional innervation to GHJ from the acromial branch of SSN was found in two specimens (figure 3A,C). As the lateral trunk coursed around the spinoglenoid notch and entered the infraspinous fossa, it gave off 1–2 articular branches that coursed directly to the joint capsule (figure 4). Regardless of the origin, the articular branches terminated in the posterosuperior capsule. The identified landmark to localize the articular branches from the lateral trunk of SSN was the suprascapular artery, in the supraspinous fossa, at the midpoint between the suprascapular and spinoglenoid notches. The suprascapular artery, veins, nerve and their branches, including articular, travel together in a neurovascular bundle, enshealthed with connective tissue. The suprascapular artery and its branches could be identified using doppler ultrasound facilitating identification of the neurovascular bundle as it passes at the midpoint of the line between the suprascapular and spinoglenoid notches.
Posteroinferior quadrant
The AN divided into anterior and posterior divisions as it coursed through the quadrangular space with the posterior circumflex humeral artery. In all 15 specimens, the posterior division, after emerging from the quadrangular space, gave off 1–3 articular branches to the posteroinferior capsule (figure 4). The articular branches coursed superiorly to reach the capsule deep to the teres minor. To localize articular branches from posterior division of AN, the junction of inferior border of teres minor and medial border of surgical neck of humerus, that is, at quadrangular space was identified as a consistent landmark.
Anterosuperior quadrant
In 14 specimens, the most superior nerve to subscapularis gave off 1–2 articular branches that coursed with the subcoracoid branch of the axillary artery, along the superior border of subscapularis, deep to the coracoid process (figure 5A,B). At the margin of the glenoid fossa, the articular branch(es) coursed deep to subscapularis to innervate the anterosuperior quadrant of GHJ. In one
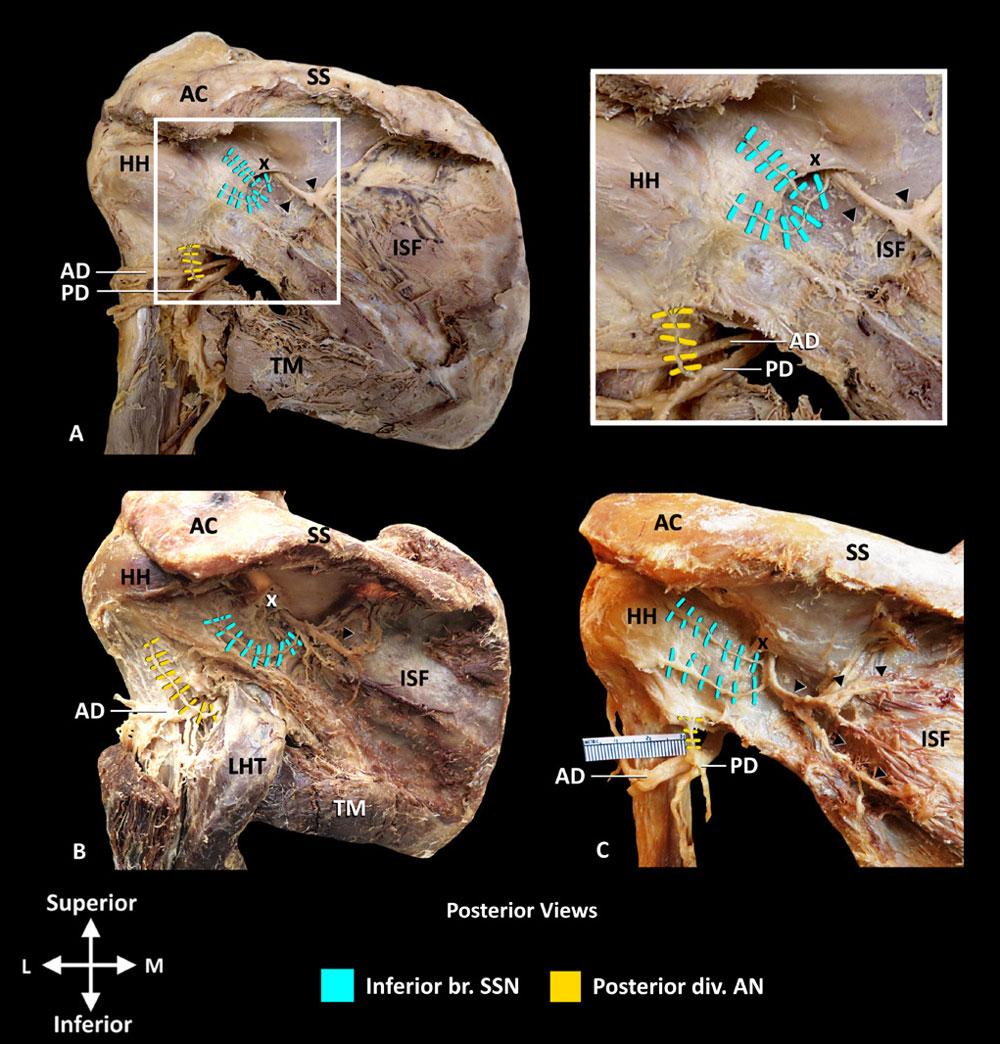
Figure 4 Nerve supply to posterior GHJ capsule. (A–C) Inferior branch of SSN and posterior division of AN. Note infraspinatus and teres minor have been removed. AC, acromion process; AD, anterior division of AN; AN, axillary nerve; br, branch; GHJ, glenohumeral joint; HH, humeral head; ISF, infraspinous fossa; LHT, long head of triceps brachii; PD, posterior division of AN; SS, spine of scapula; SSN, suprascapular nerve; TM, teres major; black arrow heads, motor branches of SSN supplying infraspinatus; x, spinoglenoid ligament. Reprinted with permission from Philip Peng Educational Series.
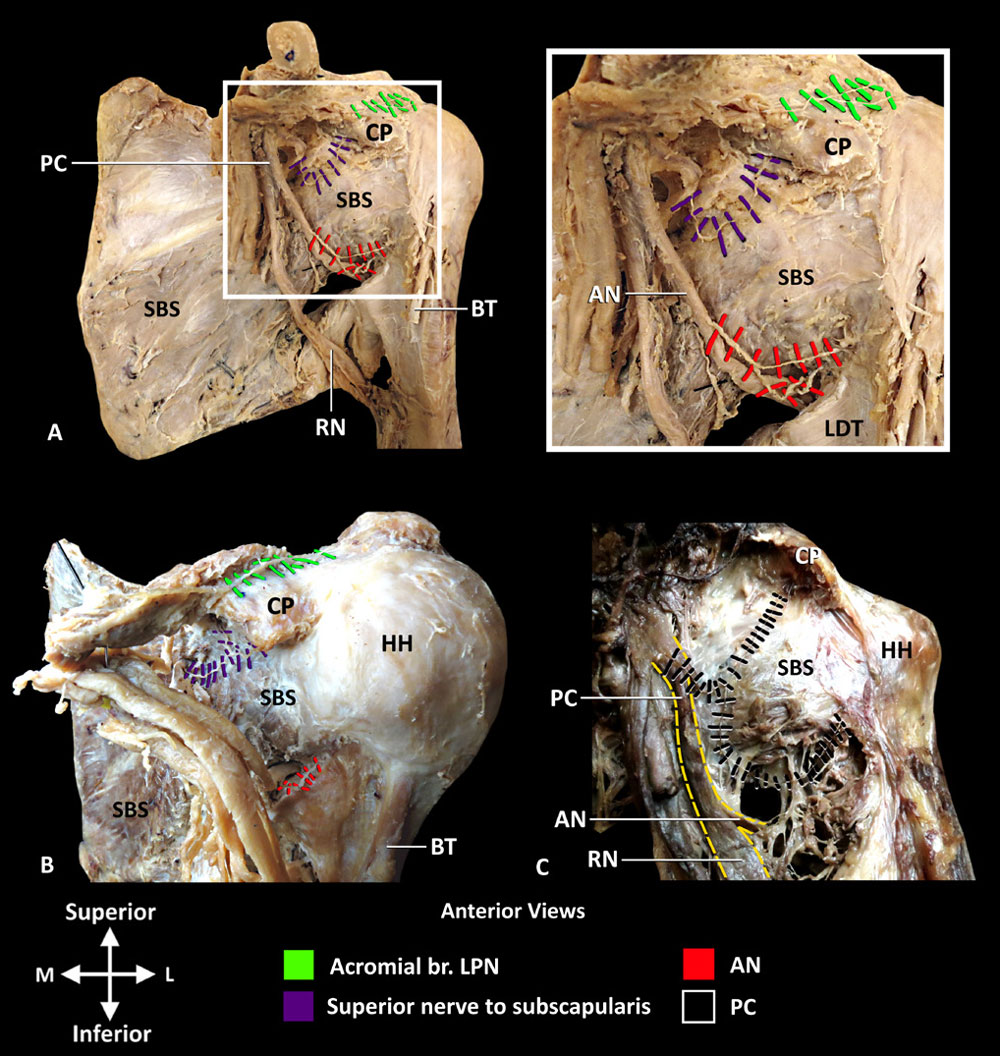
Figure 5 Innervation of ACJ and anterior GHJ capsule. (A and B) Superior nerve to subscapularis and AN innervate GHJ and lateral pectoral nerve innervates the ACJ. Inset photo is enlargement of area in white box in A. (C) Articular branches from PC innervating the GHJ. Yellow dash lines outline the PC and its bifurcation into AN and RN. ACJ, acromioclavicular joint; AN, axillary nerve; BT, tendon of long head of biceps brachii; CP, coracoid process; GHJ, glenohumeral joint; HH, humeral head; LDT, tendon of latissimus dorsi; LPN, lateral pectoral nerve; PC, posterior cord; RN, radial nerve; SBS, subscapularis. Reprinted with permission from Philip Peng Educational Series.
specimen, there were no articular branches from the nerves to subscapularis; however, the posterior cord provided an articular branch with a similar course (figure 5C). Additionally, in one specimen, the LPN gave off an articular branch to the GHJ capsule (figure 3B).
To target the articular branches supplying the anterosuperior quadrant, the superior margin of subscapularis just medial to its attachment to the lesser tubercle or the underlying margin of the rim of anterior glenoid fossa could be used. Another landmark to assist in locating the articular branches is the neurovascular bundle, containing the subcoracoid artery, veins, and articular branches of the superior nerve to subscapularis, just superior to subscapularis.
Anteroinferior quadrant
The main trunk of AN provided 1–3 articular branches to the anteroinferior quadrant (n=15) prior to its bifurcation into the anterior and posterior divisions (figure 5A,B). These branches traveled with the anterior circumflex humeral artery and coursed laterally between the tendons of subscapularis and latissimus dorsi. At the medial border of the humerus, the articular branches coursed superiorly, deep to the tendon of subscapularis, to innervation the GHJ capsule. The articular branches lie in close proximity to the junction of the inferior border of subscapularis and medial border of the surgical neck of humerus. At this location, the anterior circumflex humeral artery crosses this junction point and may be used to help localize the articular branches of the main trunk of AN.
Additionally, the following innervations were found:
► The anterior division of AN, after coursing through the quadrangular space, gave off 2–3 articular branches (n=2) that
terminated in the transverse humeral ligament (figure 6A).
► The PC and LPN gave off an articular branch, in one specimen each, that coursed on the anterior surface of subscapularis to the GHJ capsule (figures 5C and 6B).
Acromioclavicular joint
The ACJ was innervated in all 15 specimens by 1–2 articular branches of LPN and by the acromial branch of SSN. The articular branches of LPN coursed in a neurovascular bundle with the acromial branch of the thoracoacromial artery and the accompanying veins, superior to the coracoid process, prior to terminating in the joint capsule (figures 6C,D and 7A,C). The acromial branch originated from the main trunk of SSN, prior to its bifurcation, and coursed along the posterior margin of the coracoid process to the ACJ (figures 3A,C and 7A,B). The neurovascular bundle consisting of the articular branches of LPN, acromial branches of the thoracoacromial artery and corresponding veins
course anterior to the coracoclavicular ligament. This neurovascular bundle could be used to localize the articular branches. The superior border of scapula at lateral margin of the suprascapular notch could be used to locate the acromial branch of SSN.
The pattern of innervation of the GHJ was broken down by territory: posterosuperior, posteroinferior, anterosuperior, and anteroinferior quadrants (figure 2). The course of each nerve in all 15 specimens was consolidated to generate a frequency map (figure 7). The primary findings are summarized as follows:

Figure 6 Course of articular branches of anterior division of AN and LPN. (A and B). Innervation of ACJ and posteroinferior/anteroinferior quadrants of GHJ. (C and D). Innervation of ACJ by LPN. AC, acromion process; ACJ, acromioclavicular joint; AD, anterior division of AN; AN, axillary nerve; br, branch; BT, tendon of long head of biceps brachii; CL, clavicle; CP, coracoid process; div, division; GHJ, glenohumeral joint; HH, humeral head; INF, infraspinatus; LC, lateral cord; LPN, lateral pectoral nerve; MCN, musculocutaneous nerve; PD, posterior division of AN; V, acromial branches of thoracoacromial artery and vein. Reprinted with permission from Philip Peng Educational Series.

Figure 7 Frequency map of innervation of GHJ and ACJ. Numbered arrows in A–C indicate course of: (1) medial trunk of SSN; (2) motor branches of SSN supplying infraspinatus; (3) anterior and posterior divisions of AN. AC, acromion process; ACJ, acromioclavicular joint; AN, axillary nerve; BG, bicipital groove; br, branch; CL, clavicle; CP, coracoid process; div, division; GHJ, glenohumeral joint; HH, humeral head; ISF,
infraspinous fossa; LPN, lateral pectoral nerve; SBF, subscapular fossa; SGN, spinoglenoid notch; SN, suprascapular notch; SS, spine of scapula; SSF, supraspinous fossa; SSN, suprascapular nerve; *, coracoid process. Reprinted with permission from Philip Peng Educational Series.
► Posterosuperior quadrant: superiorly by articular branches of SSN in supraspinous fossa; posteriorly by articular branches of SSN in the infraspinous fossa.
► Posteroinferior quadrant: articular branches from posterior division of AN.
► Anterosuperior quadrant: articular branches from superior nerve to subscapularis.
► Anteroinferior quadrant: articular branches from main trunk of AN.
Additionally, less frequent innervation (1–2 specimens) was provided by the PC and LPN both supplying the anterosuperior and anteroinferior quadrants.
This novel cadaveric study has documented the articular branches innervating the GHJ and ACJ simultaneously in relation to bony, vascular, and soft-tissue landmarks visible with image guidance. The frequency map of articular branches provides an anatomical
basis to propose novel interventions. The innervation of GHJ, in the current study, was found to originate from SSN, AN, LPN, and NS, concurring with the previous literature. Gardner was the first to report the innervation of GHJ from all four articular branches (SSN, AN, LPN and NS). More recently, Nasu et al14 found an articular branch terminating in the transverse humeral ligament that originated from the anterior division of AN,14 and Eckmann et al16 found additional articular branches from SSN that coursed along the superior border of the spine of scapula to supply the posterosuperior GHJ capsule. However, in the current study, branches of the musculocutaeneous nerve reported by Wrete (1948) were not found. The ACJ, consistent with the previous literature, was found to be innervated by articular branches of LPN and acromial branch
of SSN. Of the four previous cadaveric studies that investigated the innervation of ACJ, two reported articular branches from both the LPN and SSN, and two other studies reported innervation from only SSN. Clinically, localization of the nerve throughout its course and identifying its relation to bony and soft tissue landmarks are important for image-guided intervention. The current study is unique in that both bony and soft tissue landmarks, visible with image guidance systems, have been defined relative to the nerves supplying the GHJ and ACJ. The frequency map, along with the dissected specimens, enable identification of bony and soft tissue landmarks relevant to all specimens included in this study. The key landmarks identified include the coracoid process, midpoint between the suprascapular and spinoglenoid notches, rim of
the anterior glenoid fossa, and acromial branch of the thoracoacromial artery. In previous studies, Nam et al reported the course of the articular branch from LPN in relation to the coracoid process and coracoclavicular ligament, and Eckmann et al related the articular branches of SSN, LPN and AN to the spine of scapula, thoracoacromial artery and head of humerus, respectively. Presently, the SSN is the main target for shoulder denervation with the suprascapular notch used as the landmark for radiofrequency cannula placement. In the current study, the articular branches of SSN innervating the posterosuperior GHJ capsule were found to originate from the lateral trunk of SSN. The motor branches of the medial and lateral trunks of SSN supply the anterior and posterior regions of supraspinatus, respectively. In a follow-up study, Kim et al suggested that the anterior region is responsible for shoulder abduction while the posterior region plays a greater role in joint stabilization. Therefore, lesioning the SSN at the suprascapular notch would likely capture both medial and lateral trunks resulting in paralysis of supraspinatus. If the medial trunk could be spared, shoulder
abduction may be preserved. This may be possible by targeting the midpoint between the suprascapular and spinoglenoid notches, the location where articular branches originate from the lateral trunk (n=13/15 specimens). The posterior region of supraspinatus and infraspinatus would be denervated; however, other external rotators of the GHJ are preserved, that is, those innervated by AN. Clinically, while sparing of the medial trunk is theoretically possible in this area, clinical correlation is required to assess the motor effects of radiofrequency or other methods of ablation in this area. Similarly, the articular branches originating from LPN could be targeted with ultrasound using the coracoid process and the accompanying acromial branch of the thoracoacromial artery located anterior to the coracoclavicular ligament. The articular branches from AN and NS would be more challenging to capture as they lie adjacent to major motor nerves or neurovascular bundles. Further studies are needed to investigate the feasibility of the RFA approaches. Additionally, peripheral nerve stimulation, a more recent technique that has been used to treat chronic shoulder pain, requires accurate lead placement with precise knowledge of anatomical landmarks.
Based on the results of the current study, AN stimulation likely targets the capsular nerves supplying the inferior half of the GHJ, whereas SSN stimulation may target the posterosuperior quadrant. In the context of perioperative pain management, there
has been much discussion and research regarding the optimal block(s) for shoulder surgery. Interscalene block (ISB) is most commonly performed; however, ISB targets the brachial plexus resulting in both sensory and motor blockade of the upper limb. Similarly, alternative approaches such as the suprascapular nerve block and the combined suprascapular and axillary nerve block (SSAXB) targets mixed nerves which results in sensory and motor blockade. In the current study, the localization of articular branches supplying the GHJ and ACJ capsules have been related to bony and soft tissue landmarks. This anatomical data provides anesthesiologists with the knowledge to develop novel blocks that mainly target the sensory afferents to the GHJ and ACJ thereby minimizing motor blockade.
For example, pericapsular infiltration of local anesthetic deep to subscapularis could cover the articular branches from the AN and NS supplying the anteroinferior/superior quadrants of GHJ. This information may provide insight for the reason and
possible solution to the residual anterior shoulder pain following SSAXB. A limitation of this study includes the small sample size (n=15). Sample size calculation was not possible due to lack of previous data. In the absence of previous data, Julious justified a sample size of as being appropriate for a pilot study. Additionally, a small sample size may not be reflective of all anatomical variations in innervation pattern. However, the frequency map compiled in the current study suggests the innervation pattern is consistent. In conclusion, the capsular distribution of articular branches, supplying the GHJ can be defined by quadrants. The posterosuperior quadrant consistently received innervation from the SSN; posteroinferior from posterior division of AN; anterosuperior
from superior nerve of subscapularis; and anteroinferior from the main trunk of AN. The ACJ was innervated in all specimen by the LPN and acromial branch of SSN. Detailed knowledge of the innervation of the GHJ and ACJ are important for planning and optimizing perioperative analgesia and shoulder RFA procedures.
Acknowledgements The authors wish to thank Ian Bell, Trevor Robinson, and Harun Bola for their valuable technical assistance. We also wish to thank the individuals who donate their bodies and tissue for the advancement of education and research.
Contributors JT, PWHP and AMRA are guarantors. JT is a PhD candidate who is supervised by AMRA and PWHP. Therefore, as mandated by the School of Graduate Studies at the University of Toronto, the supervisors must be actively involved in all aspects of the research including literature search, experimental design, collecting and analyzing data, interpreting results, and manuscript preparation.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests PWHP received equipment support from Sonosite Fujifilm Canada.
Patient consent for publication Not required.
Ethics approval University of Toronto Health Sciences Research Ethics Board (#27210).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement This is a cadaveric study and all data/findings have been reported in the manuscript.
United States Bone and Joint Initiative. The burden of musculoskeletal diseases in the United States (BMUS). 3rd edn. Rosemont, IL:
1. United States Bone and Joint Initiative, 2014.
2 HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality, 2011.
3 Day JS, Lau E, Ong KL, et al. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg 2010;19:1115–20.
4 Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the
United States. J Bone Joint Surg Am 2011;93:2249–54.
5 Bjørnholdt KT, Brandsborg B, Søballe K, et al. Persistent pain is common 1-2 years
after shoulder replacement. Acta Orthop 2015;86:71–7.
6 Rohof OJ. Radiofrequency treatment of peripheral nerves. Pain Pract 2002;2:257–60.
7 Gardner E. The innervation of the shoulder joint. Anat Rec 1948;102:1–18.
8 Wrete M. The innervation of the shoulder-joint in man. Cells Tissues Organs 1949;7:173–90.
9 Aszmann OC, Dellon AL, Birely BT, et al. Innervation of the human shoulder joint and
its implications for surgery. Clin Orthop Relat Res 1996;330:202–7.
10 Akita K, Kawashima T, Shimokawa T, et al. Cutaneous nerve to the subacromial region originating from the lateral pectoral nerve. Ann Anat 2002;184:15–19.
11 Gelber PE, Reina F, Monllau JC, et al. Innervation patterns of the inferior glenohumeral ligament: anatomical and biomechanical relevance. Clin Anat 2006;19:304–11.
12 Vorster W, Lange CP, Briët RJ, et al. The sensory branch distribution of the
suprascapular nerve: an anatomic study. J Shoulder Elbow Surg 2008;17:500–2.
13 Ebraheim NA, Whitehead JL, Alla SR, et al. The suprascapular nerve and its articular branch to the acromioclavicular joint: an anatomic study. J Shoulder Elbow Surg 2011;20:e13–e17.
14 Nasu H, Nimura A, Yamaguchi K, et al. Distribution of the axillary nerve to the
subacromial bursa and the area around the long head of the biceps tendon. Knee
Surg Sports Traumatol Arthrosc 2015;23:2651–7.
15 Nam Y-S, Panchal K, Kim I-B, et al. Anatomical study of the articular branch of the
lateral pectoral nerve to the shoulder joint. Knee Surg Sports Traumatol Arthrosc
2016;24:3820–7.
16 Eckmann MS, Bickelhaupt B, Fehl J, et al. Cadaveric study of the articular branches of the shoulder joint. Reg Anesth Pain Med 2017;42:564–70.
17 Shah RV, Racz GB. Pulsed mode radiofrequency lesioning of the suprascapular nerve for the treatment of chronic shoulder pain. Pain Physician 2003;6:503–6.
18 Liliang PC, Lu K, Liang CL, et al. Pulsed radiofrequency lesioning of the suprascapular nerve for chronic shoulder pain: a preliminary report. Pain Med 2009;10:70–5.
19 Gofeld M, Restrepo-Garces CE, Theodore BR, et al. Pulsed radiofrequency of
suprascapular nerve for chronic shoulder pain: a randomized double-blind active
placebo-controlled study. Pain Practice 2013;13:96–103. on 31 January 2019 by guest. Protected by copyright. file:/ Regional Anesthesia & Pain Medicine: first published as 10.1136/rapm-2018-100152 on 11 January 2019. Downloaded from Tran J, et al. Reg Anesth Pain Med 2019;0:1–7. doi:10.1136/rapm-2018-100152 7 Original article
20 Simopoulos TT, Nagda J, Aner MM. Percutaneous radiofrequency lesioning of the
suprascapular nerve for the management of chronic shoulder pain: a case series. J
Pain Res 2012;5:91–7.
21 Hermenegildo JA, Roberts SL, Kim SY. Innervation pattern of the suprascapular nerve within supraspinatus: a three-dimensional computer modeling study. Clin Anat
2014;27:622–30.
22 Kim S, Bleakney R, Boynton E, et al. Investigation of the static and dynamic
musculotendinous architecture of supraspinatus. Clin Anat 2010;23:48–55.
23 Gofeld M, Agur A. Peripheral nerve stimulation for chronic shoulder pain: a proof of
concept anatomy study. Neuromodulation 2018;21:284–9.
24 Price DJ. The shoulder block: a new alternative to interscalene brachial plexus
blockade for the control of postoperative shoulder pain. Anaesth Intensive Care
2007;35:575–81.
25 Dhir S, Sondekoppam RV, Sharma R, et al. A Comparison of Combined
Suprascapular and Axillary Nerve Blocks to Interscalene Nerve Block for Analgesia
in Arthroscopic Shoulder Surgery: An Equivalence Study. Reg Anesth Pain Med
2016;41:564–71.
26 Price D. Optimizing the Combined Suprascapular and Axillary Nerve (SSAX) Block. Reg Anesth Pain Med 2017;42:122.
27 Marty P, Rontes O, Delbos A. A comparison of combined suprascapular and axillary
nerve blocks to interscalene block: interpret With Caution. Reg Anesth Pain Med
2017;42:273–4.
28 Hussain N, Goldar G, Ragina N, et al. Suprascapular and interscalene nerve block
for shoulder surgery: A systematic review and meta-analysis. Anesthesiology
2017;127:998–1013.
29 Wiegel M, Moriggl B, Schwarzkopf P, et al. Anterior suprascapular nerve block versus interscalene brachial plexus block for shoulder surgery in the outpatient setting:
a randomized controlled patient- and assessor-blinded trial. Reg Anesth Pain Med
2017;42:310–8.
30 Zhou C, Choi S, Brull R, et al. Anterior suprascapular nerve block versus interscalene brachial plexus block for shoulder surgery in the outpatient setting. Reg Anesth Pain Med 2018;43:99–100.
31 Neuts A, Stessel B, Wouters PF, et al. Selective suprascapular and axillary nerve block versus interscalene plexus block for pain control after arthroscopic shoulder surgery: a noninferiority randomized parallel-controlled clinical trial. Reg Anesth Pain Med 2018;43:738–44.
32 Winnie AP. Interscalene brachial plexus block. Anesthesia Analgesia
1970;49:455???466–66.
33 Price DJ, Axillary PDJ. Axillary (circumflex) nerve block used in association with
suprascapular nerve block for the control of pain following total shoulder joint
replacement. Reg Anesth Pain Med 2008;33:280–1.
34 Price D. How many nerves supply the shoulder? Reg Anesth Pain Med 2018;43:334.
35 Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat
2005;4:287–91.
